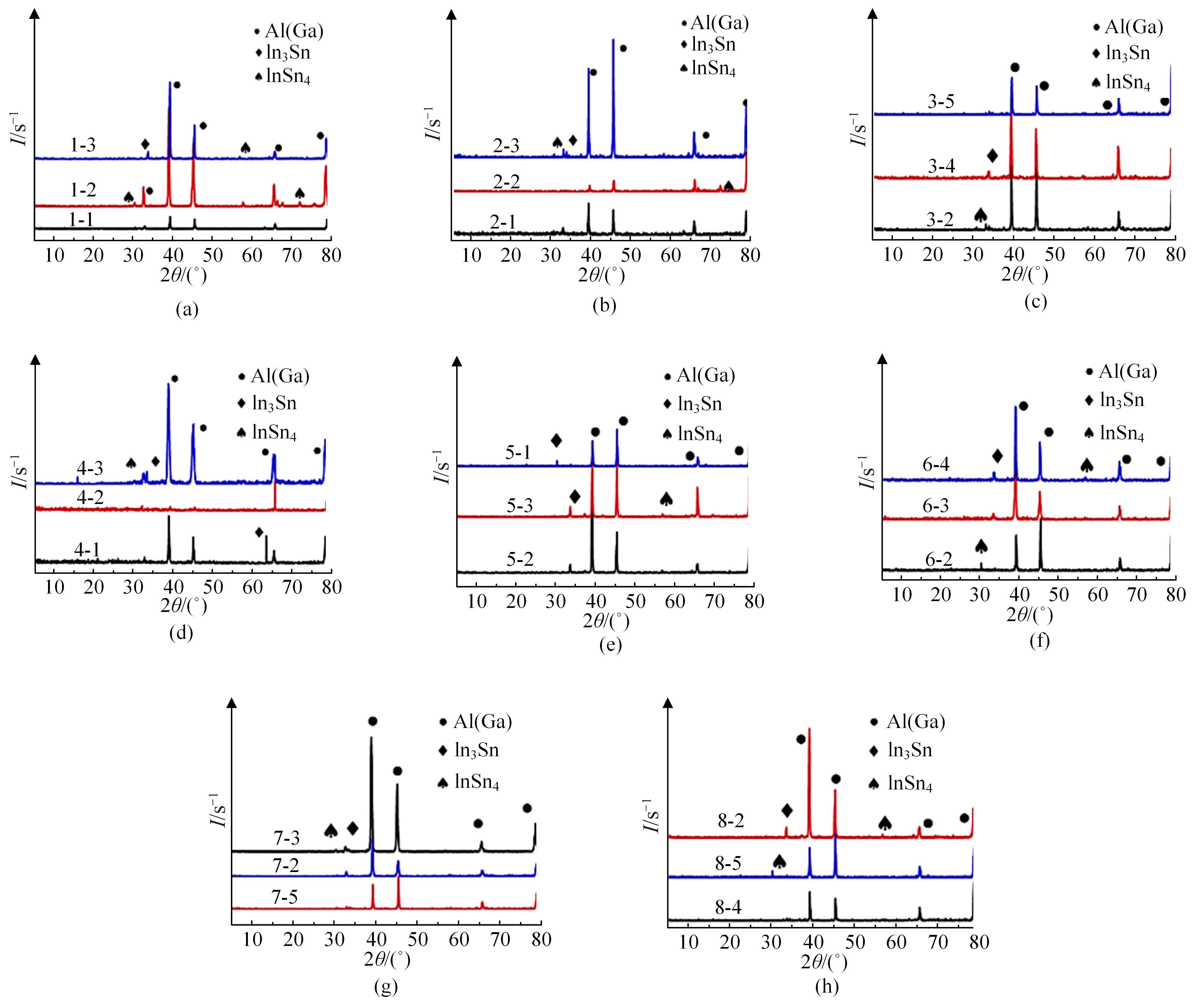
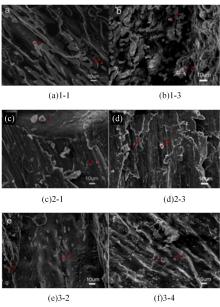
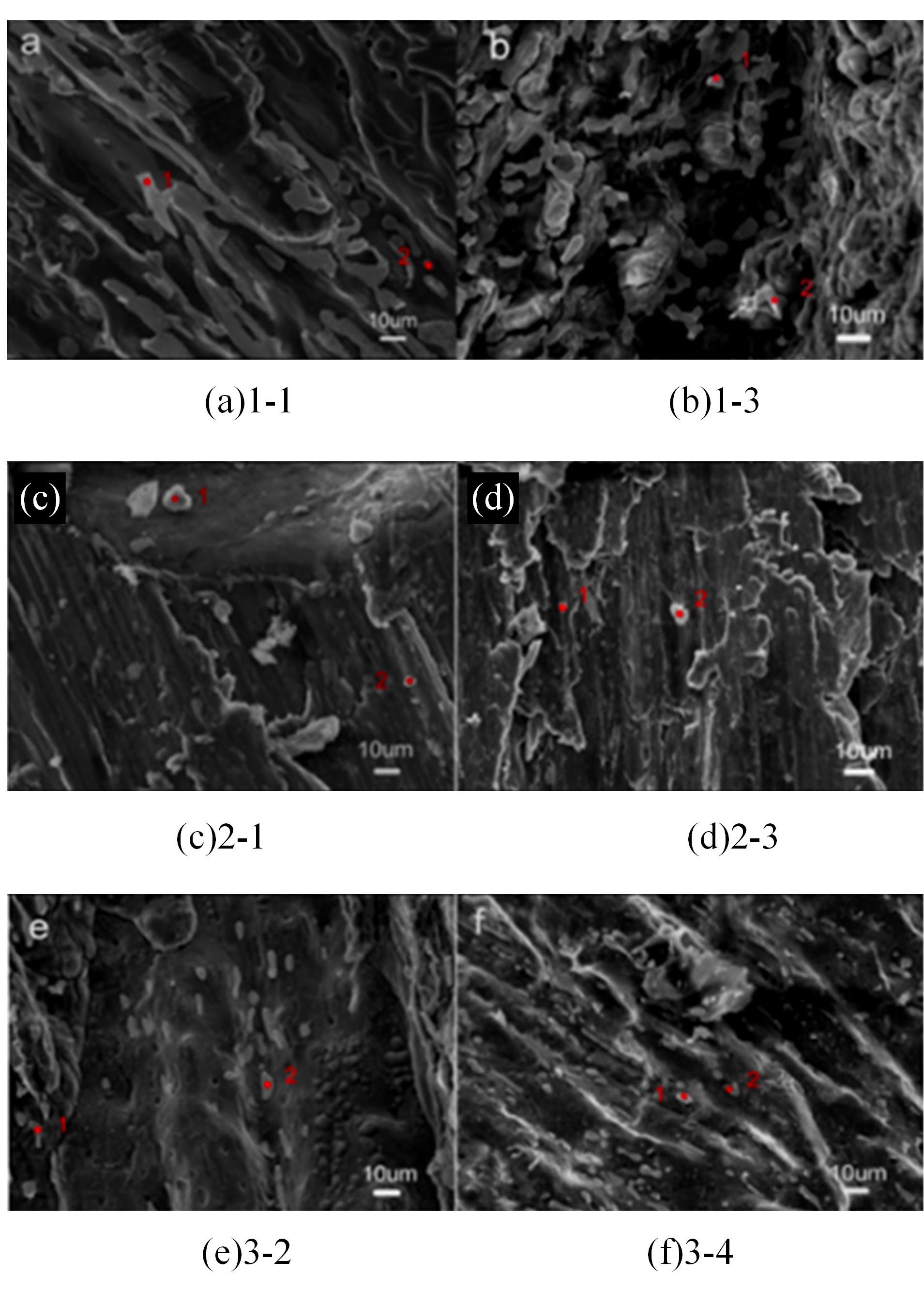
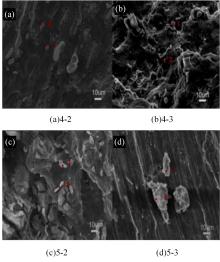
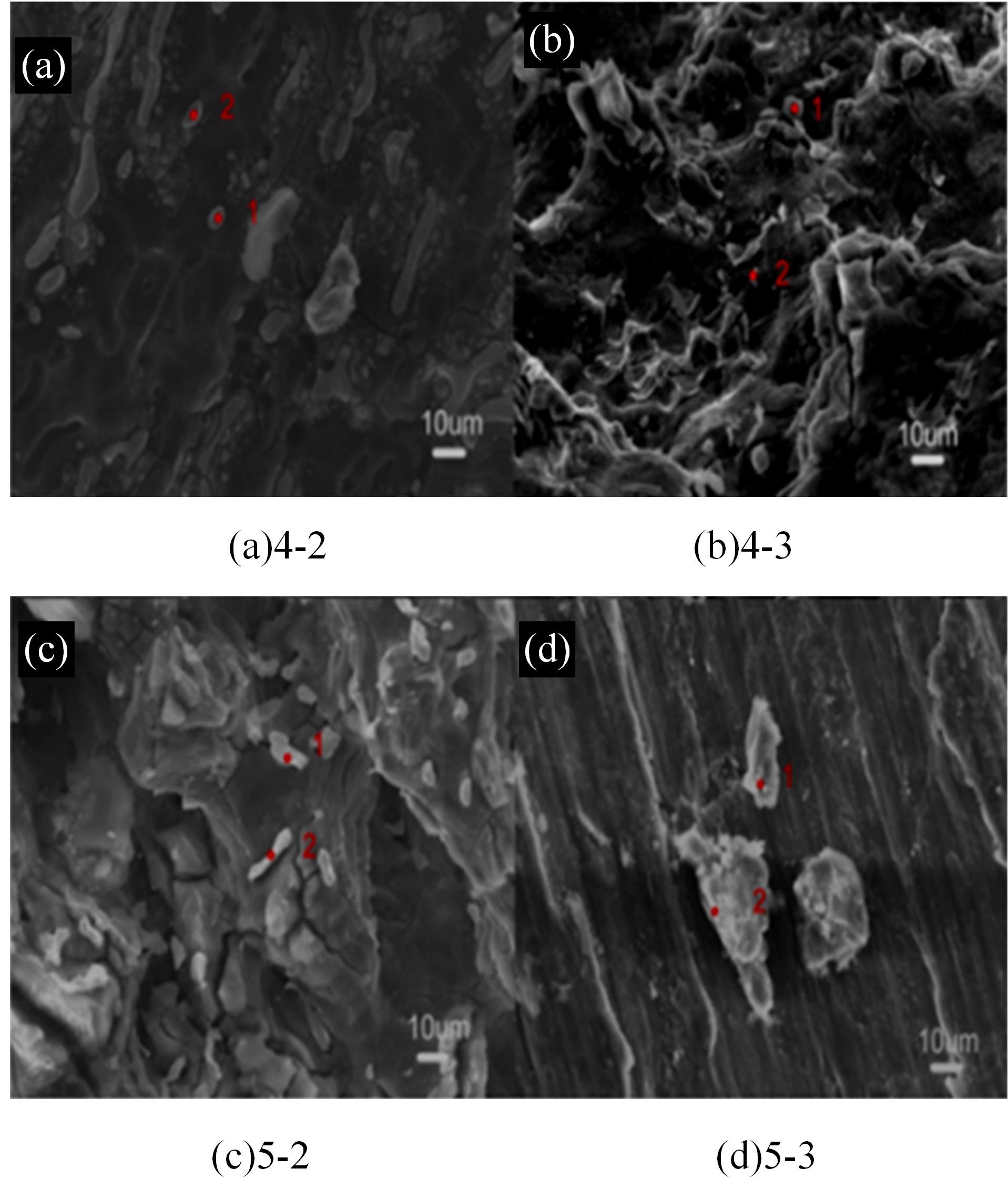
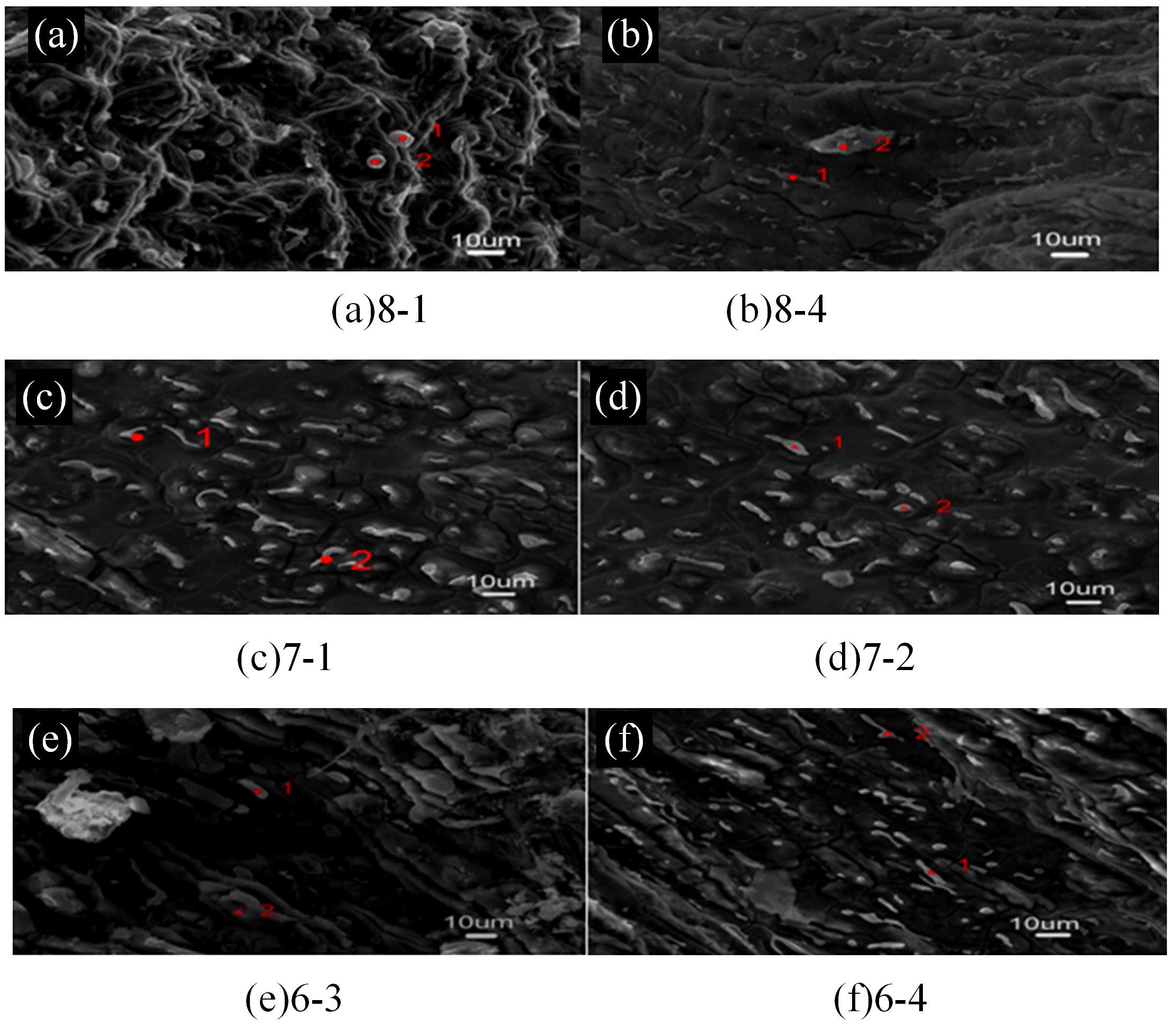
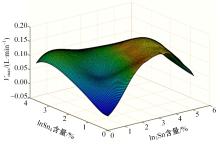
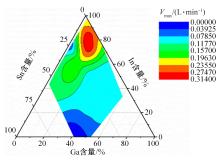
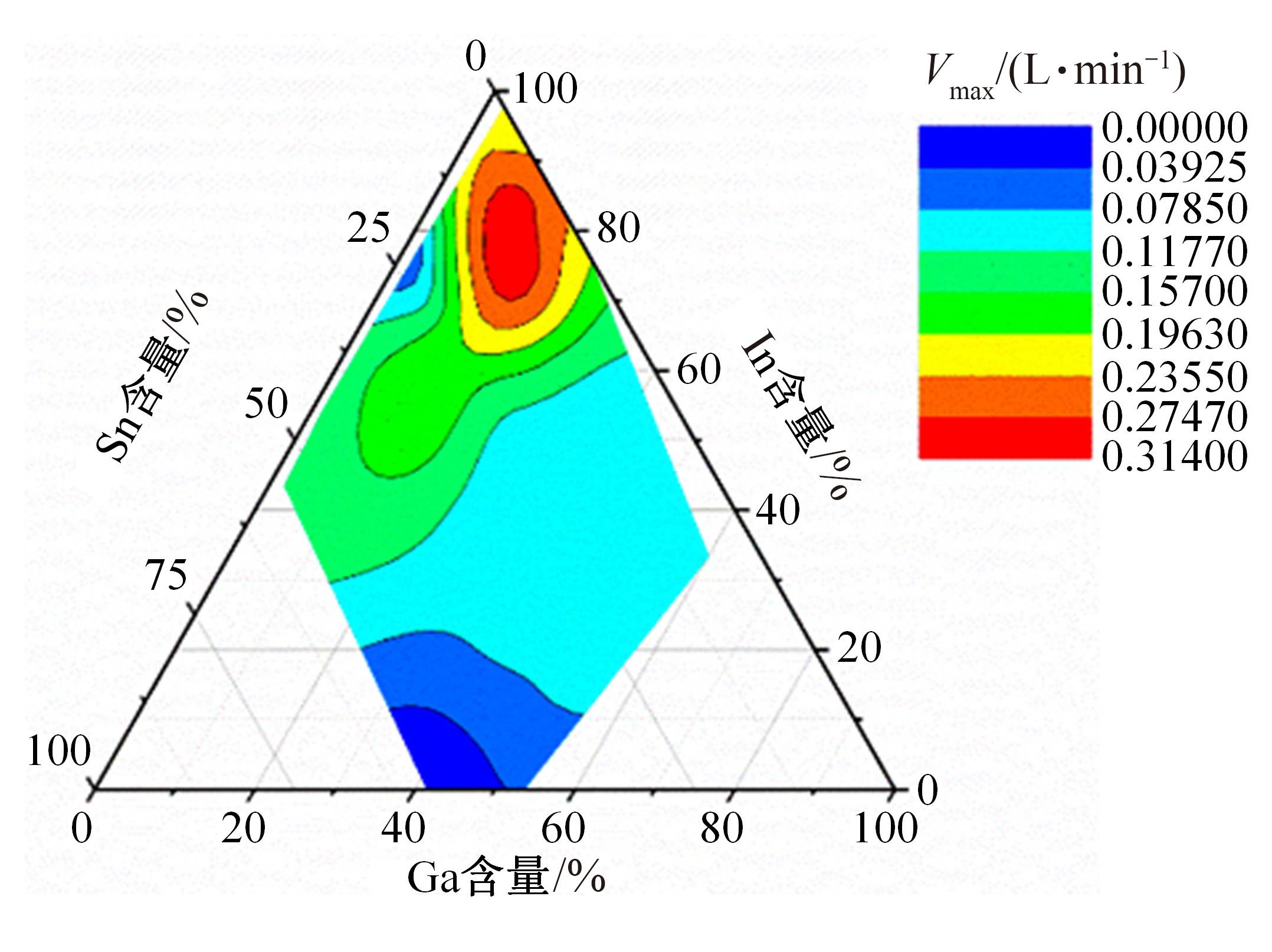
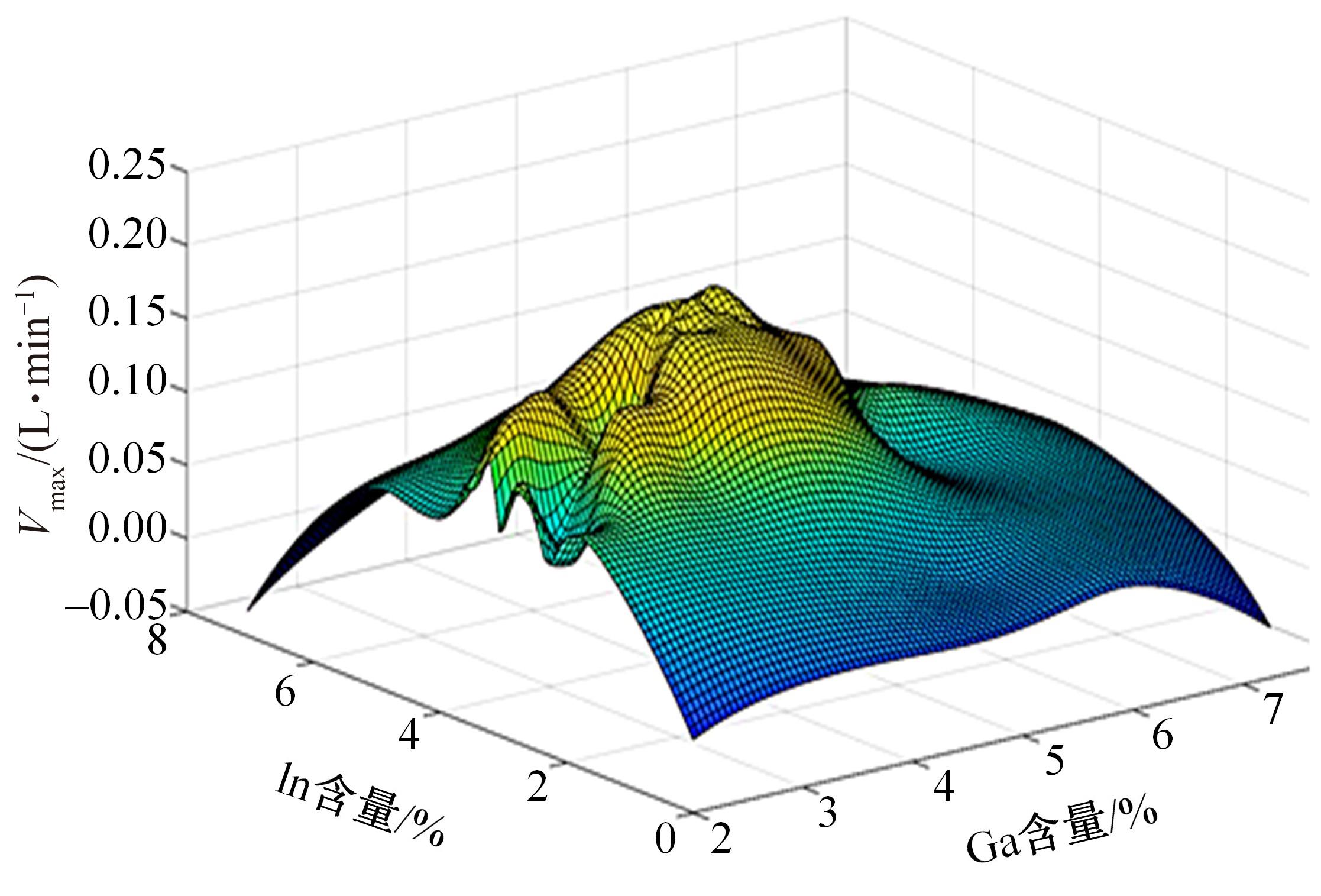
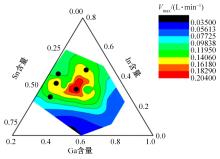
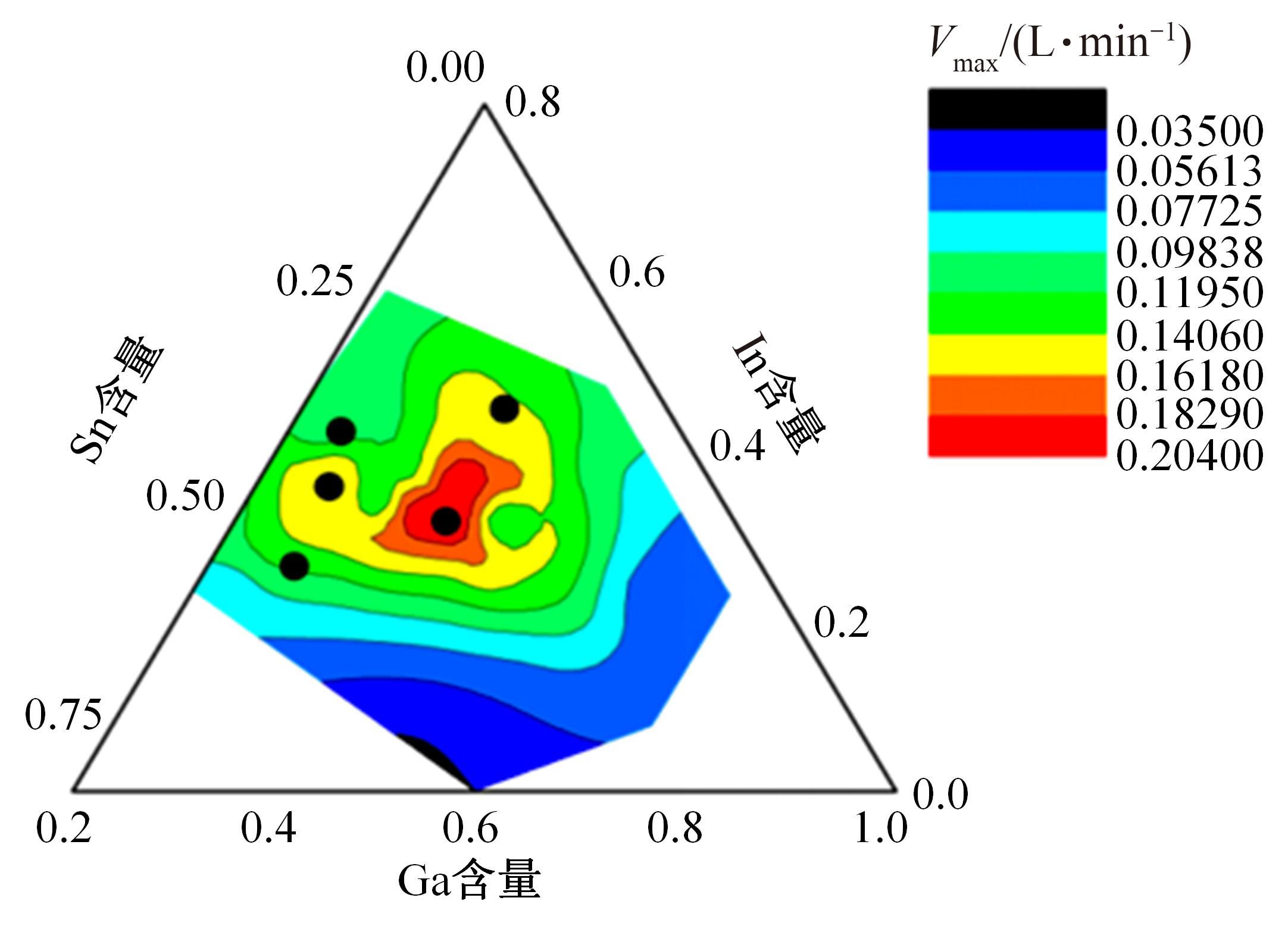
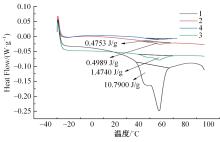
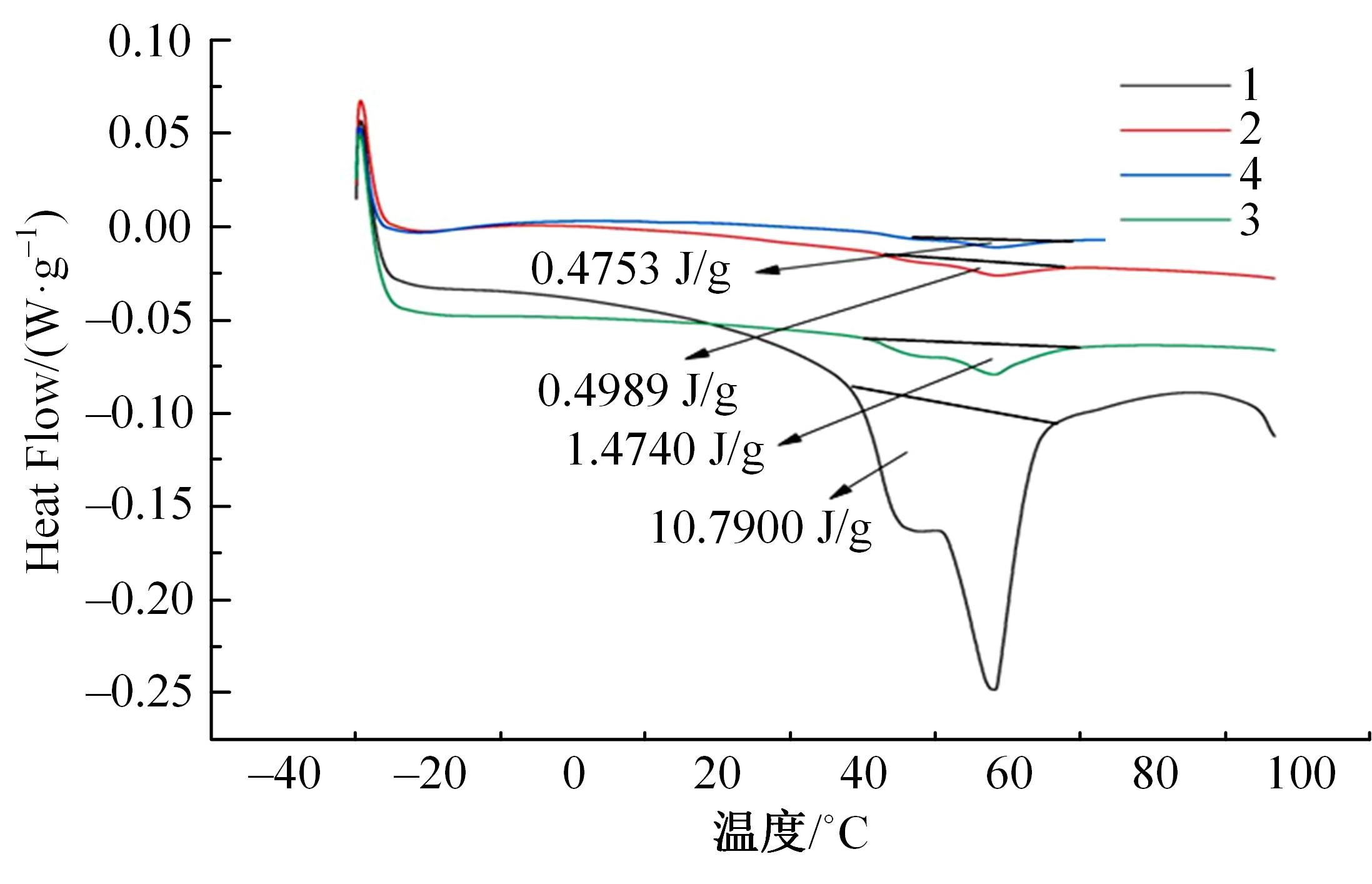
Journal of Jilin University(Engineering and Technology Edition) ›› 2024, Vol. 54 ›› Issue (1): 114-123.doi: 10.13229/j.cnki.jdxbgxb.20220218
Control method on hydrogen production rate of aluminum gallium indium tin alloy
Qian GAO1,2( ),Dong-han LI1,Zhi-jiang JIN1,Jie SHI1
),Dong-han LI1,Zhi-jiang JIN1,Jie SHI1
- 1.College of Materials Science and Engineering,Jilin University,Changchun 130022,China
2.State Key Laboratory of Automotive Simulation and Control,Jilin University,Changchun 130022,China
CLC Number:
- TS912.3
| 1 | Berger M, Goldfarb J L. Understanding our energy footprint: undergraduate chemistry laboratory investigation of environmental impacts of solid fossil fuel wastes[J]. Journal of Chemical Education, 2017, 94(8): 1124-1128. |
| 2 | Europe Oil-Telegram Group. Statistical review of world energy 2016[J]. Europe Oil-Telegram, 2016,54(48/49): 9-11. |
| 3 | Xu S, Zhao X, Liu J. Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature[J]. Renewable And Sustainable Energy Reviews, 2018, 92: 17-37. |
| 4 | Gai W Z, Liu W H, Deng Z Y, Et al. Reaction of al powder with water for hydrogen generation under ambient condition[J]. International Journal of Hydrogen Energy, 2012, 37: 13132-13140. |
| 5 | Yavor Y, Goroshin S, Bergthorson J M, et al. Comparative reactivity of industrial metal powders with water for hydrogen production[J]. International Journal of Hydrogen Energy, 2015, 40: 1026-1036. |
| 6 | Eom K S, Kwon J Y, Kim M J, et al. Design of al-fe alloys for fast on-board hydrogen production from hydrolysis[J]. Journal of Materials Chemistry, 2011, 21: 13047-13051. |
| 7 | Eom K S, Kim M J, Oh S K, et al. Design of ternary al-sn-fe alloy for fast on-board hydrogen production, and its application to pem fuel cell[J]. International Journal of Hydrogen Energy, 2011, 36: 11825-11831. |
| 8 | Nithiya A, Saffarzadeh A, Shimaoka T. Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (Mswi) Bottom ash[J]. Waste Management, 2018, 73: 342-350. |
| 9 | Chen X Y, Zhao Z W, Hao M M, et al. Research of hydrogen generation by the reaction of al-based materials with water[J]. Journal of Power Sources, 2013, 222: 188-195. |
| 10 | Kravchenko O V, Semenenko K N, Bulychev B M, et al. Activation of aluminum metal and its reaction with water[J]. Journal of Alloys and Compounds, 2005, 397: 58-62. |
| 11 | Wang H Z, Leung D Y C, Leung M K H, et al. A review on hydrogen production using aluminum and aluminum alloys[J]. Renewable and Sustainable Energy Reviews, 2009, 13: 845-853. |
| 12 | Zou H B, Chen S Z, Zhao Z H, et al. Hydrogen production by hydrolysis of aluminum[J]. Journal of Alloys and Compounds, 2013, 578: 380-384. |
| 13 | Cuomo J J, Woodall J M. Solid state renewable energy supply[P]. US: 4358291. |
| 14 | Ziebarth T, Woodall J M, Kramer R A, et al. Liquid phase-enabled reaction of a1-ga and A1-Ga-In-Sn alloys with water[J]. International Journal of Hydrogen Energy, 2011, 36: 5271-5279. |
| 15 | Wang W, Zhao X M, Chen D M, et al. Insight into the reactivity of Al-Ga-In-Sn alloy with water[J]. International Journal of Hydrogen Energy, 2012, 37: 2187-2194. |
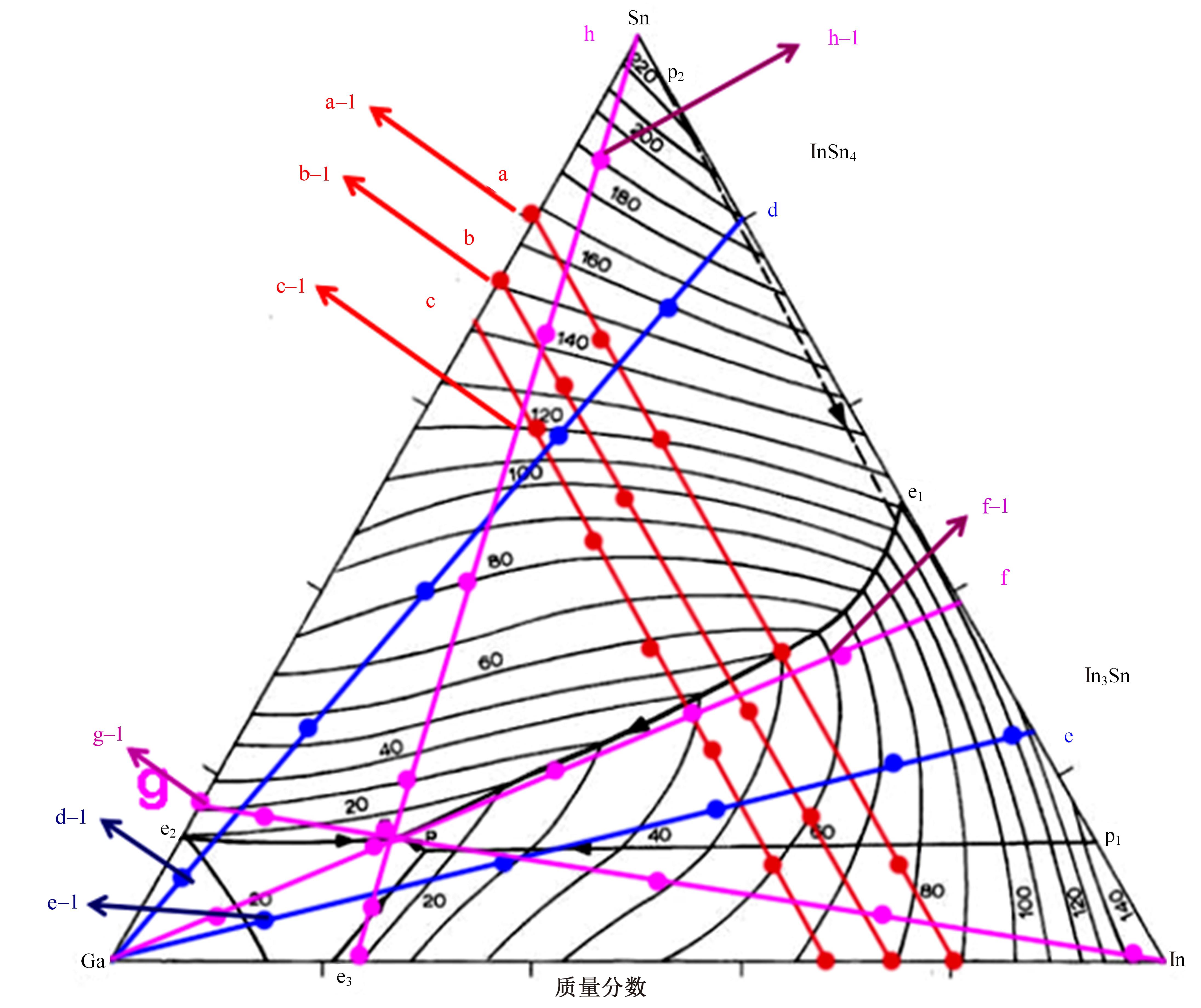
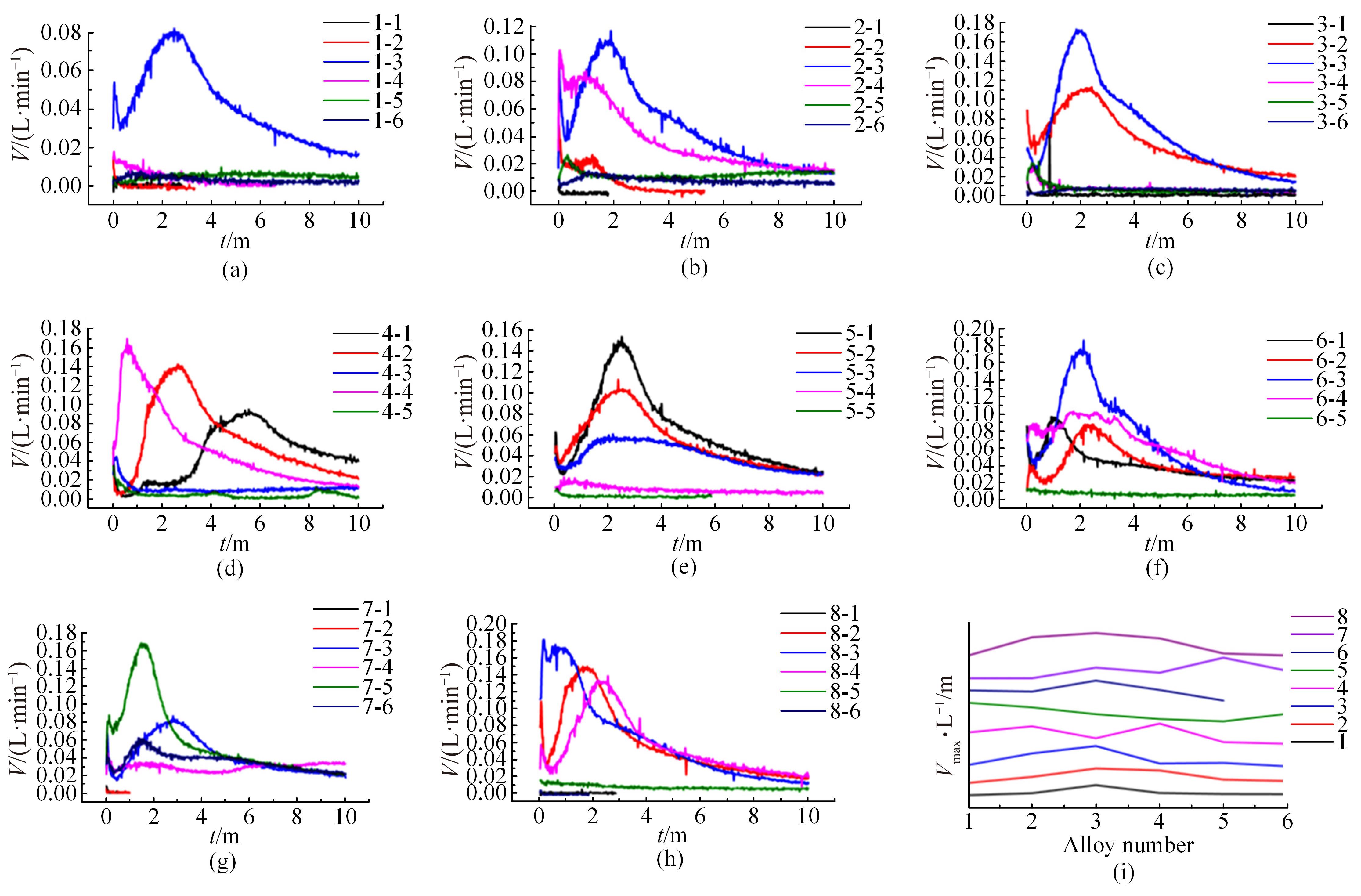
| 16 | He T T, Wang W, Chen W, et al. Influence of in and sn compositions on the reactivity of Al-Ga-In-Sn alloys with water[J]. International Journal of Hydrogen Energy, 2017, 42: 5627-5637. |
| 17 | 贺甜甜. 活性铝合金-水体系产氢性能及机理研究[D]. 北京:中国科学院大学, 2015. |
| He Tian-tian. Hydrogen production performance and mechanism of activated aluminum alloy-water system[D]. Beijing:University of Chinese Academy of Sciences, 2015. | |
| 18 | He T T, Wang W, Chen W, et al. Influence of in and sn compositions on the reactivity of Al-Ga-In-Sn alloys with water[J]. International Journal of Hydrogen Energy, 2017, 42: 5627-5637. |
| 19 | 刘昕, 吴天祥, 赵群丽 等. 基于Kriging代理模型对产不饱和脂肪酸的酒曲微生物混菌比例优化[J]. 酿酒科技, 2016(9): 23-27. |
| Liu Xin, Wu Tian-xiang, Zhao Qun-li, et al. Optimization of mixed ratio of microbial strains to produce unsaturated fatty acids based on kriging model[J]. Liquor-making Science & Technology, 2016(9): 23-27. |
| No related articles found! |
|
||