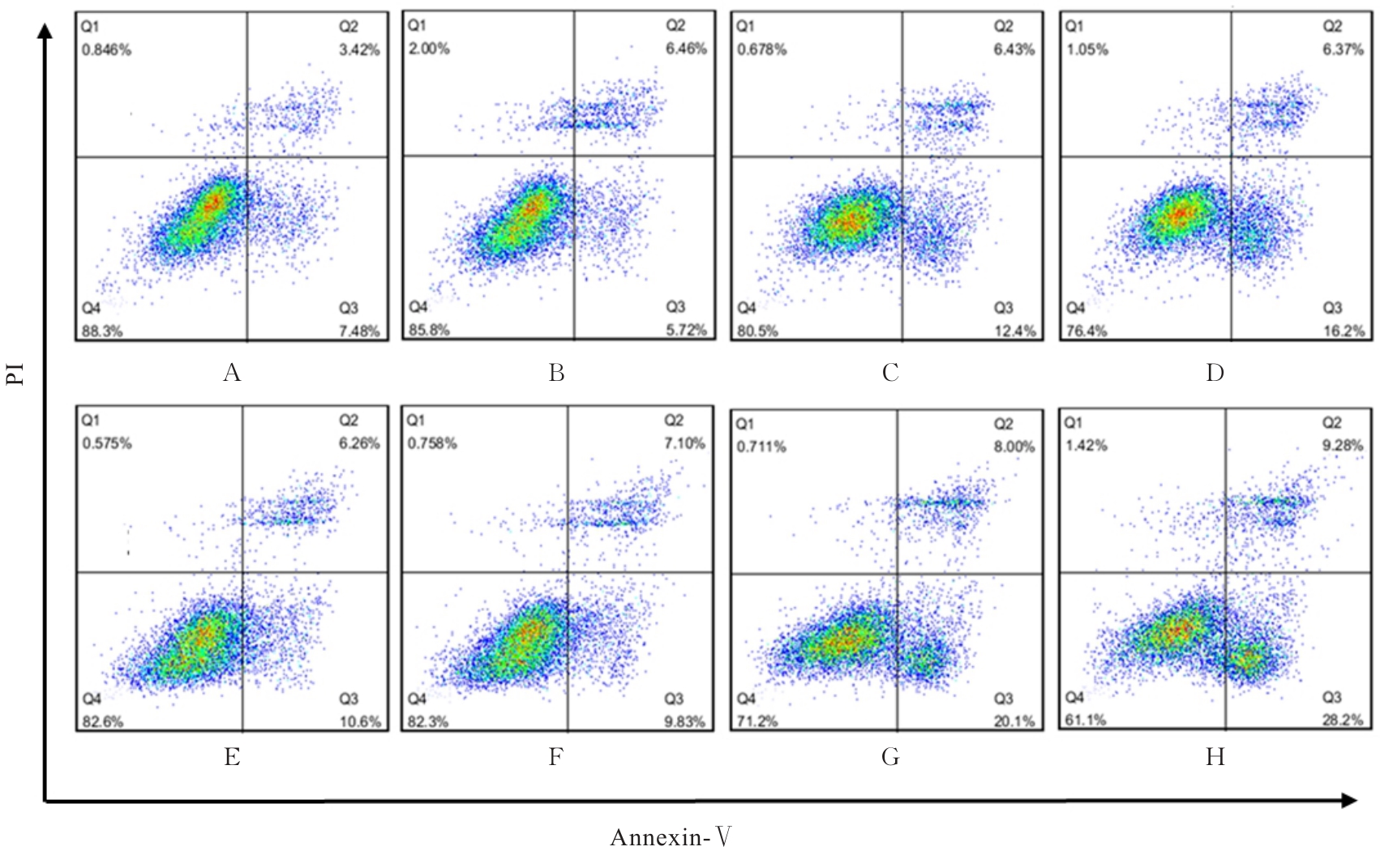
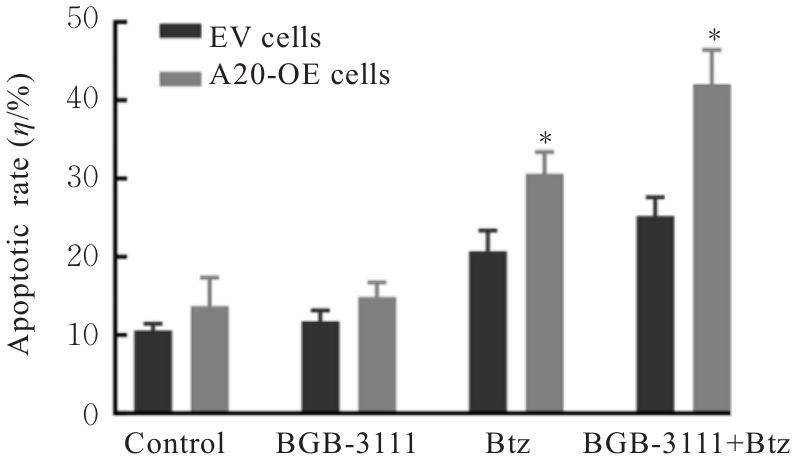
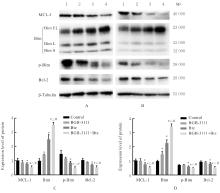
| [1] |
HANAMURA I. Multiple myeloma with high-risk cytogenetics and its treatment approach[J]. Int J Hematol, 2022, 115(6): 762-777.
|
| [2] |
GULLA A, MORELLI E, SAMUR M K, et al. Bortezomib induces anti-multiple myeloma immune response mediated by cGAS/STING pathway activation[J]. Blood Cancer Discov, 2021, 2(5): 468-483.
|
| [3] |
王亚茹, 马艳萍. 多发性骨髓瘤硼替佐米耐药机制的研究进展[J]. 中国实验血液学杂志, 2023, 31(5): 1584-1587.
|
| [4] |
BAI Y, SU X. Updates to the drug-resistant mechanism of proteasome inhibitors in multiple myeloma[J]. Asia-Pac J Clncl Oncology, 2021, 17(1): 29-35.
|
| [5] |
VO J N, WU Y M, MISHLER J, et al. The genetic heterogeneity and drug resistance mechanisms of relapsed refractory multiple myeloma[J]. Nat Commun, 2022, 13(1): 3750.
|
| [6] |
ZHOU Q, WANG H Y, SCHWARTZ D M, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease[J]. Nat Genet, 2016, 48(1): 67-73.
|
| [7] |
ELBAZ O, SHAAT R M, GHAFFAR H AABD EL, et al. The prevalence of MYD88 L265P and TNFAIP3 mutations and their correlations with clinico-hematological profile in Egyptian patients with diffuse large B cell lymphoma[J]. Asian Pac J Cancer Prev, 2023, 24(7): 2485-2491.
|
| [8] |
YANG C Z, ZANG W C, TANG Z F, et al. A20/TNFAIP3 regulates the DNA damage response and mediates tumor cell resistance to DNA-damaging therapy[J]. Cancer Res, 2018, 78(4): 1069-1082.
|
| [9] |
GUO Q D, DONG H, LIU X N, et al. A20 is overexpressed in glioma cells and may serve as a potential therapeutic target[J]. Expert Opin Ther Targets, 2009, 13(7): 733-741.
|
| [10] |
DONG B F, LV G Y, WANG Q, et al. Targeting A20 enhances TRAIL-induced apoptosis in hepatocellular carcinoma cells[J]. Biochem Biophys Res Commun, 2012, 418(2): 433-438.
|
| [11] |
CODD J D, SALISBURY J R, PACKHAM G, et al. A20 RNA expression is associated with undifferentiated nasopharyngeal carcinoma and poorly differentiated head and neck squamous cell carcinoma[J]. J Pathol, 1999, 187(5): 549-555.
|
| [12] |
MALYNN B A, MA A. A20 takes on tumors: tumor suppression by an ubiquitin-editing enzyme[J]. J Exp Med, 2009, 206(5): 977-980.
|
| [13] |
AMMANN E M, SHANAFELT T D, WRIGHT K B, et al. Updating survival estimates in patients with chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL) based on treatment-free interval length[J]. Leuk Lymphoma, 2018, 59(3): 643-649.
|
| [14] |
MOHAMED A J, YU L, BÄCKESJÖ C M, et al. Bruton’s tyrosine kinase (btk): function, regulation, and transformation with special emphasis on the PH domain[J]. Immunol Rev, 2009, 228(1): 58-73.
|
| [15] |
THOMPSON P A, BURGER J A. Bruton’s tyrosine kinase inhibitors: first and second generation agents for patients with Chronic Lymphocytic Leukemia (CLL)[J]. Expert Opin Investig Drugs, 2018, 27(1): 31-42.
|
| [16] |
SMITH C I, BASKIN B, HUMIRE-GREIFF P, et al. Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK is selectively down-regulated in T lymphocytes and plasma cells[J]. J Immunol, 1994, 152(2): 557-565.
|
| [17] |
HERMAN S E M, MONTRAVETA A, NIEMANN C U, et al. The bruton tyrosine kinase (BTK) inhibitor acalabrutinib demonstrates potent on-target effects and efficacy in two mouse models of chronic lymphocytic leukemia[J]. Clin Cancer Res, 2017, 23(11): 2831-2841.
|
| [18] |
WU J J, LIU C, TSUI S T, et al. Second-generation inhibitors of Bruton tyrosine kinase[J]. J Hematol Oncol, 2016, 9(1): 80.
|
| [19] |
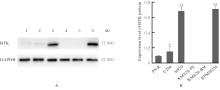
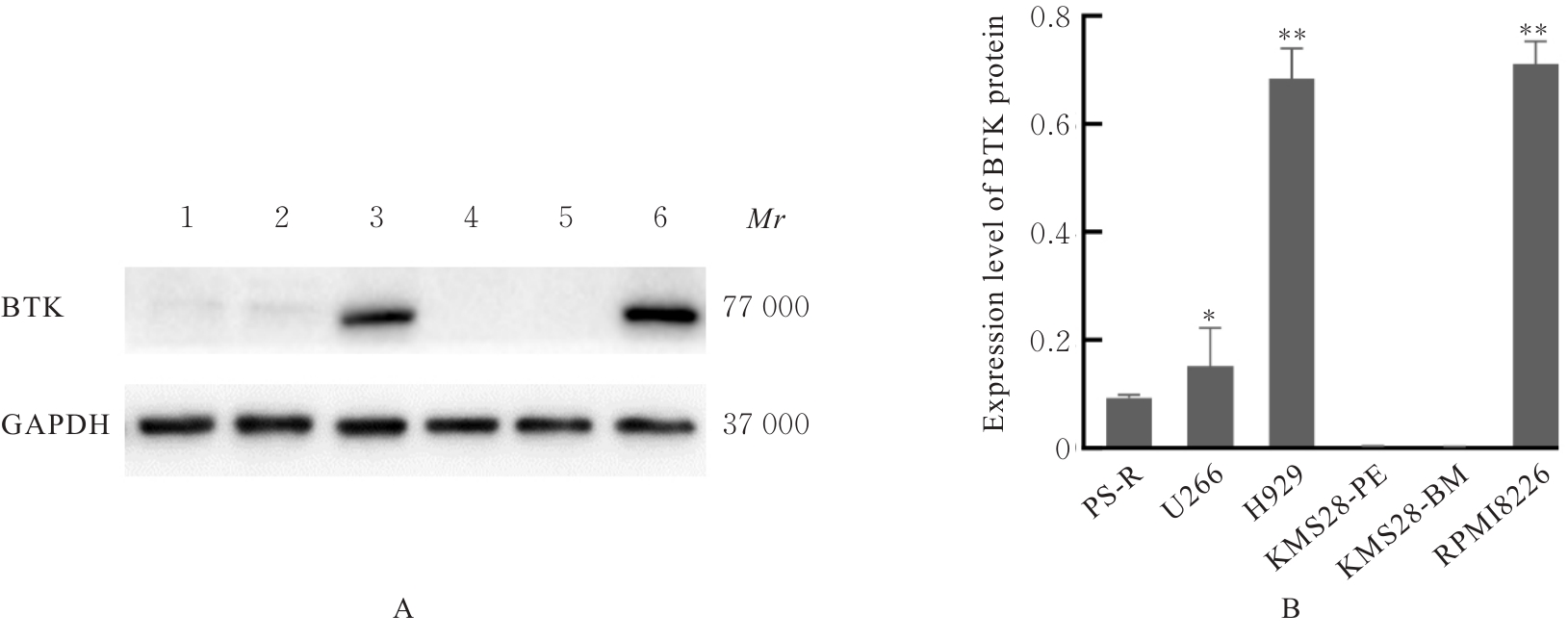
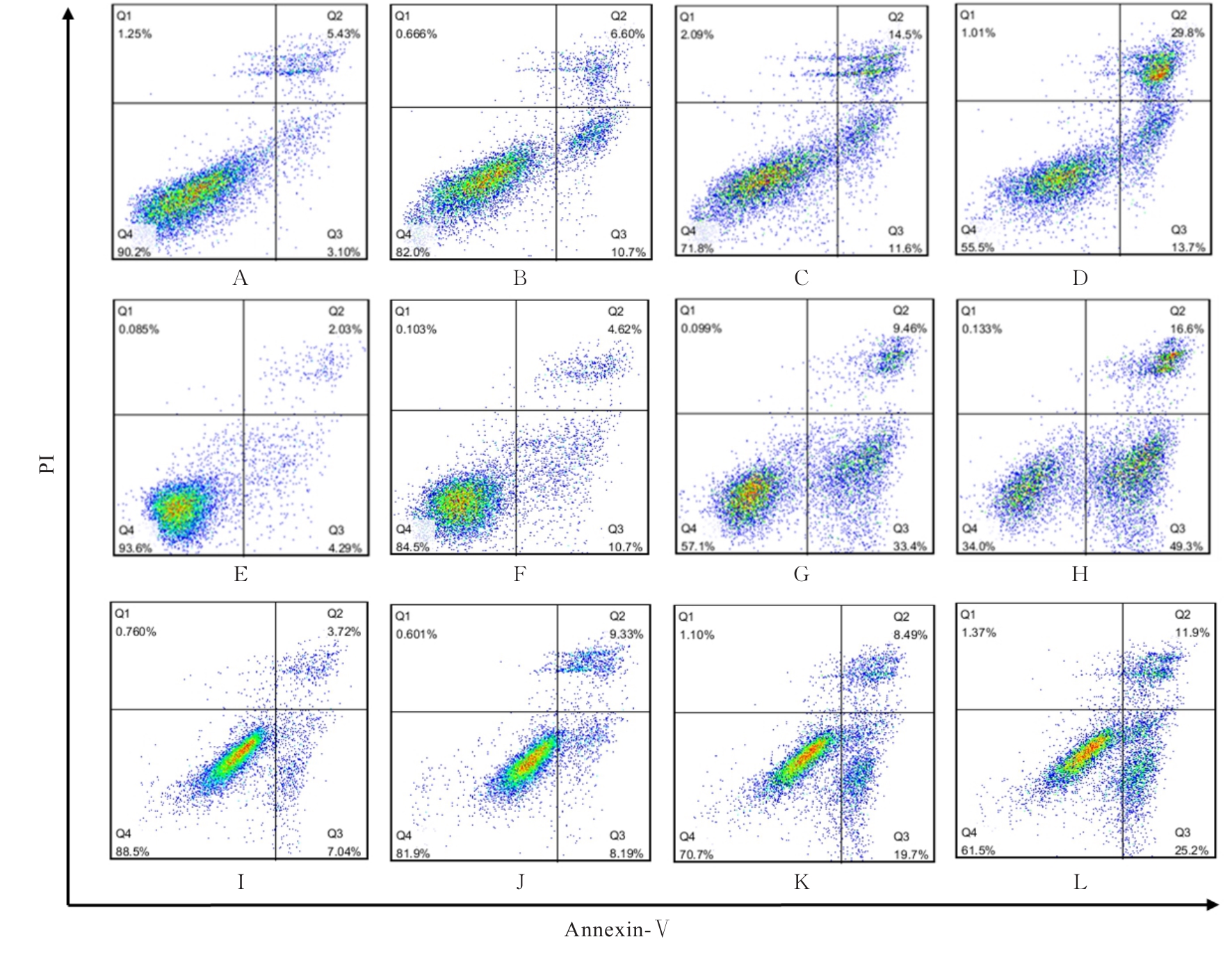
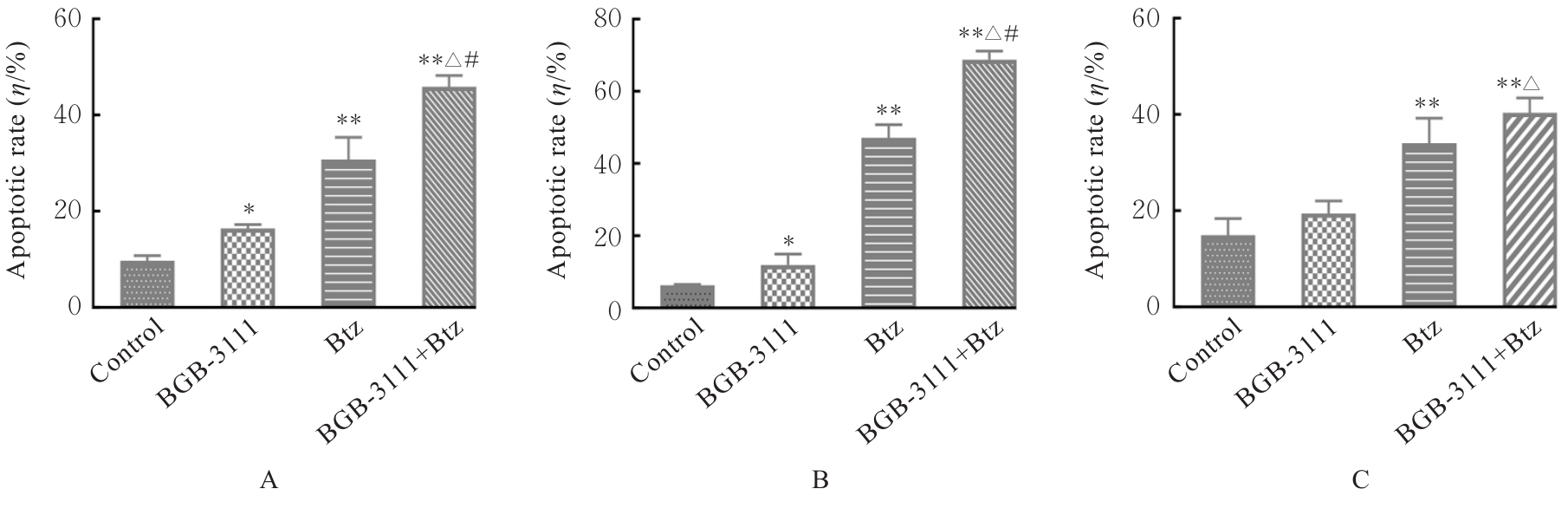
贾晓辉. BTK在套细胞淋巴瘤中表达的临床意义及其抑制剂BGB-3111联合硼替佐米的协同抗肿瘤效应[D]. 天津: 天津医科大学, 2017.
|
| [20] |
BURLEY T A, KENNEDY E, BROAD G, et al. Targeting the non-canonical NF-κB pathway in chronic lymphocytic leukemia and multiple myeloma[J]. Cancers (Basel), 2022, 14(6): 1489.
|
| [21] |
KALTSCHMIDT B, WITTE K E, GREINER J F W, et al. Targeting NF-κB signaling in cancer stem cells: a narrative review[J]. Biomedicines, 2022, 10(2): 261.
|
| [22] |
ANDERSON K C. Proteasome inhibitors in multiple myeloma[J]. Semin Oncol, 2009, 36(2 ): S20-S26.
|
| [23] |
LORK M, VERHELST K, CYLDBEYAERT R, A20 and OTULIN deubiquitinases in NF-κB signaling and cell death: so similar, yet so different[J]. Cell Death Differ, 2017, 24(7): 1172-1183.
|
| [24] |
MOONEY E C, SAHINGUR S E. The ubiquitin system and A20: implications in health and disease[J]. J Dent Res, 2021, 100(1): 10-20.
|
| [25] |
SCHMITZ R, HANSMANN M L, BOHLE V, et al. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma[J]. J Exp Med, 2009, 206(5): 981-989.
|
| [26] |
KIM S W, RAMASAMY K, BOUAMAR H, et al. microRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20)[J]. Proc Natl Acad Sci USA, 2012, 109(20): 7865-7870.
|
| [27] |
LIM M C C, MAUBACH G, BIRKL-TOEGLHOFER A M, et al. A20 undermines alternative NF-κB activity and expression of anti-apoptotic genes in Helicobacter pylori infection[J]. Cell Mol Life Sci, 2022, 79(2): 102.
|
 )
)