吉林大学学报(医学版) ›› 2023, Vol. 49 ›› Issue (6): 1473-1483.doi: 10.13481/j.1671-587X.20230610
PPC/PBS引导骨再生生物膜的制备及其理化性能和生物学特征评价
- 吉林大学口腔医院种植科,吉林 长春 130021
Preparation of PPC/PBS-based guided bone regeneration membrane and evaluation of its physiochemical properties and biological characteristics
Xiaolu SHI,Ye TIAN,Shaobo ZHAI,Yang LIU,Shunli CHU( )
)
- Department of Prosthodontics,Stomatology Hospital,Jilin University,Changchun 130021,China
摘要:
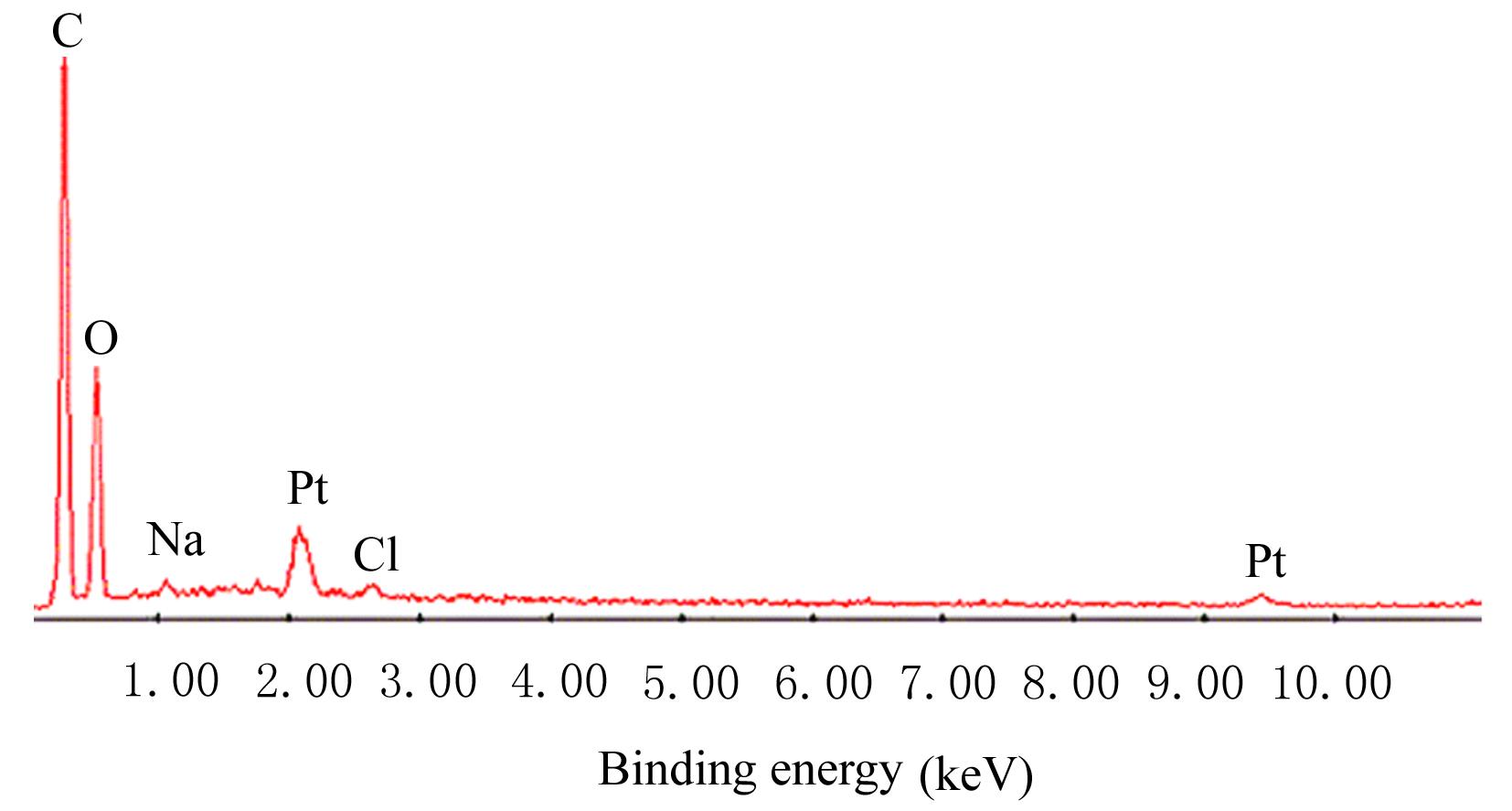
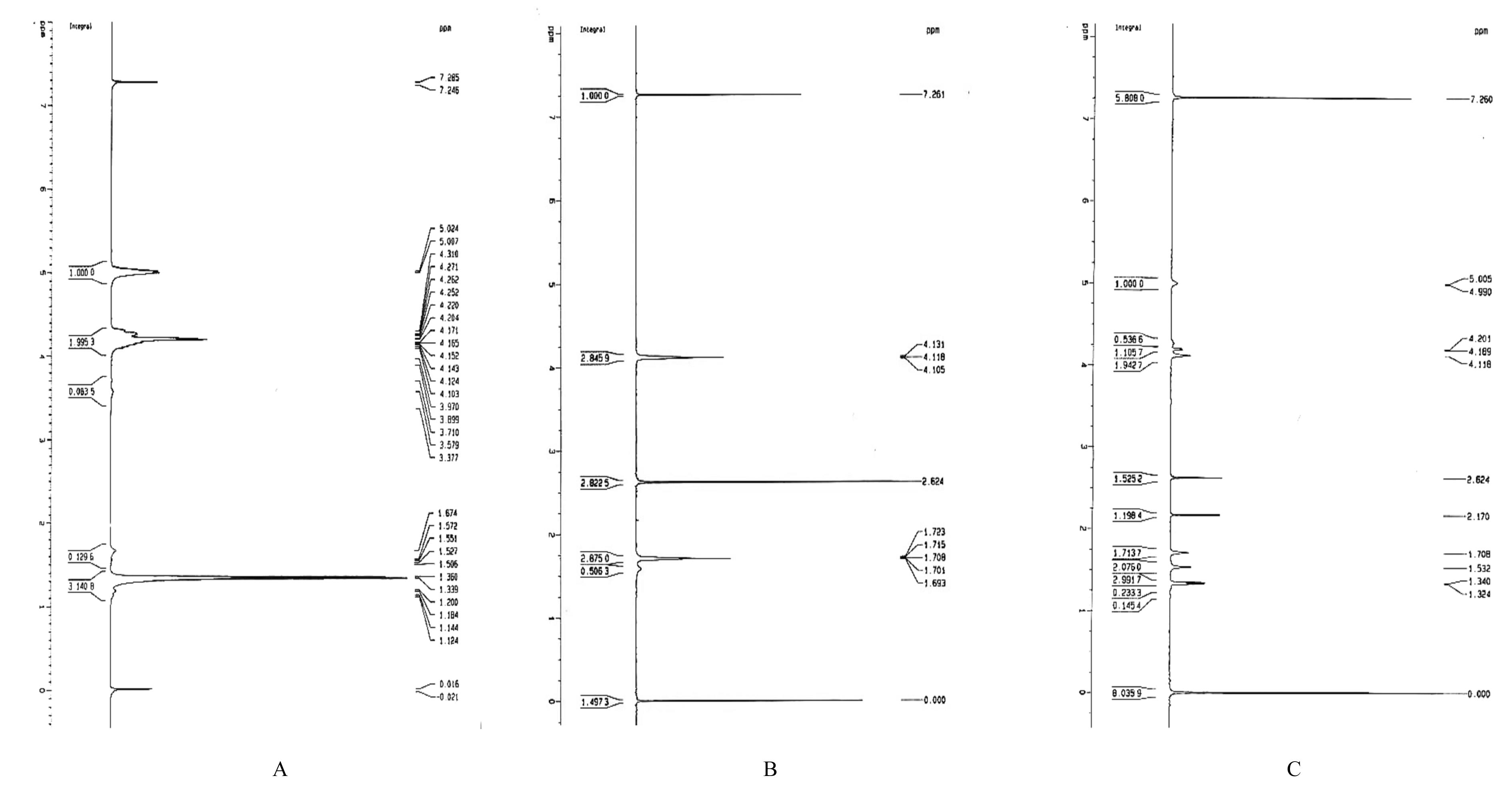
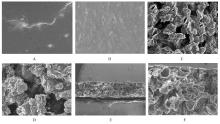
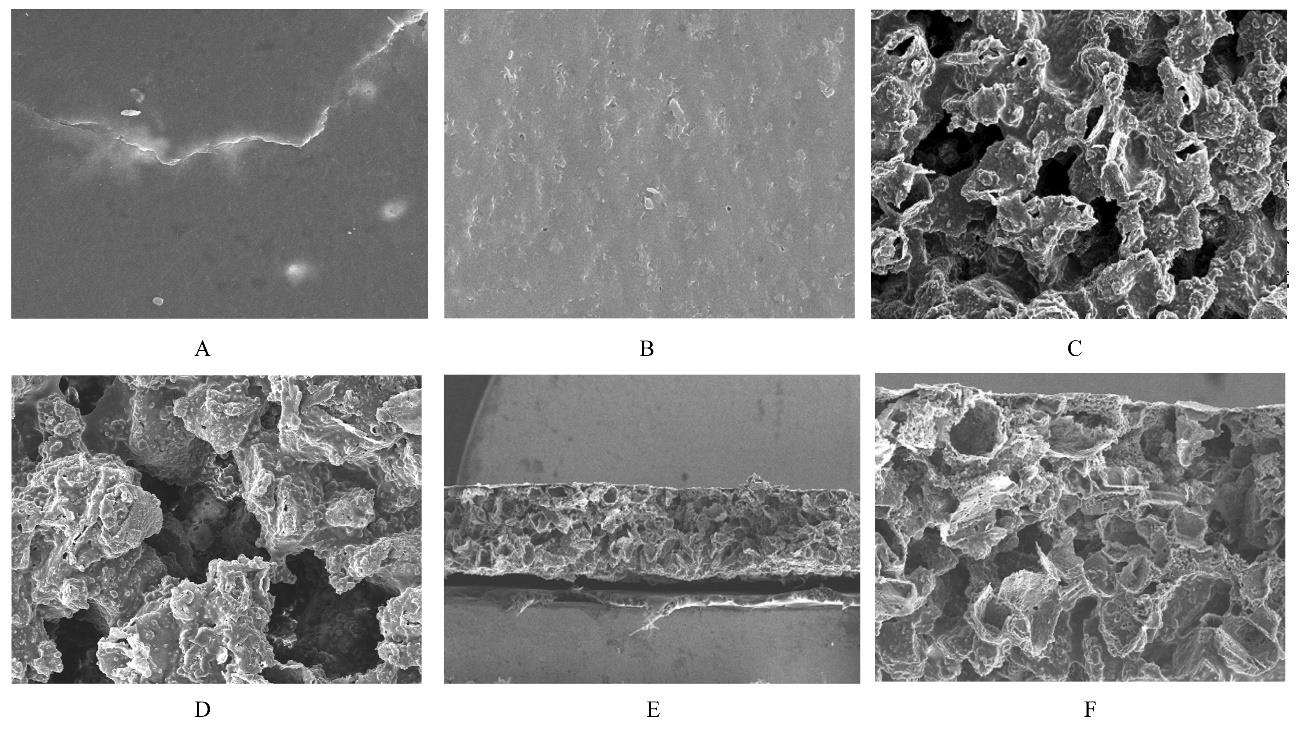
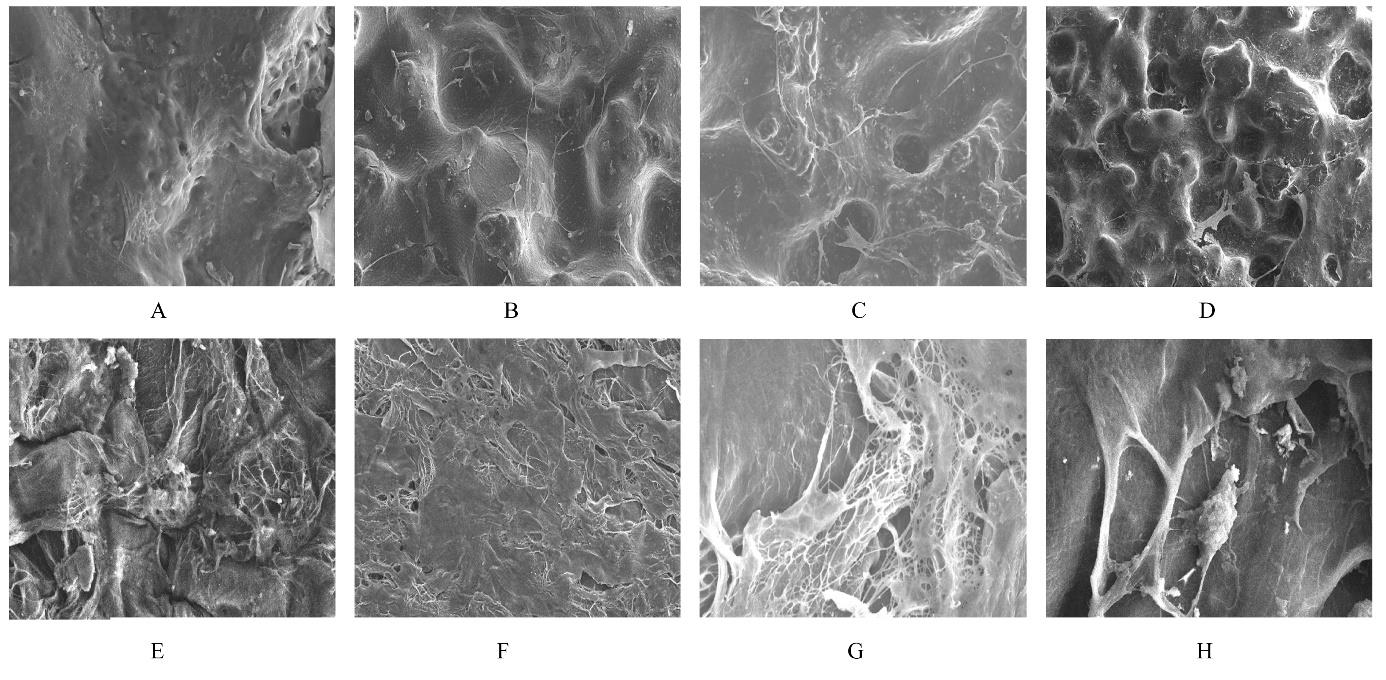
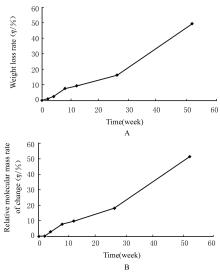
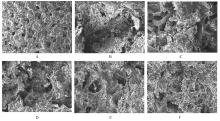
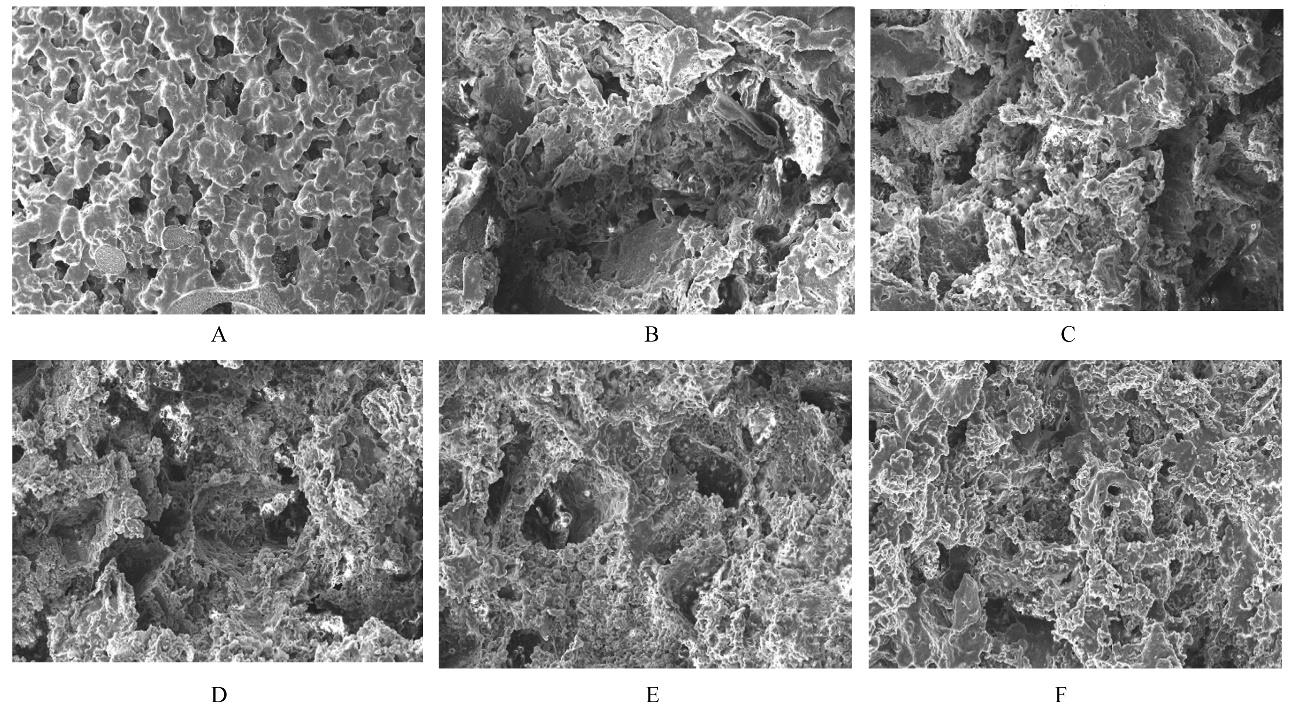
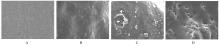
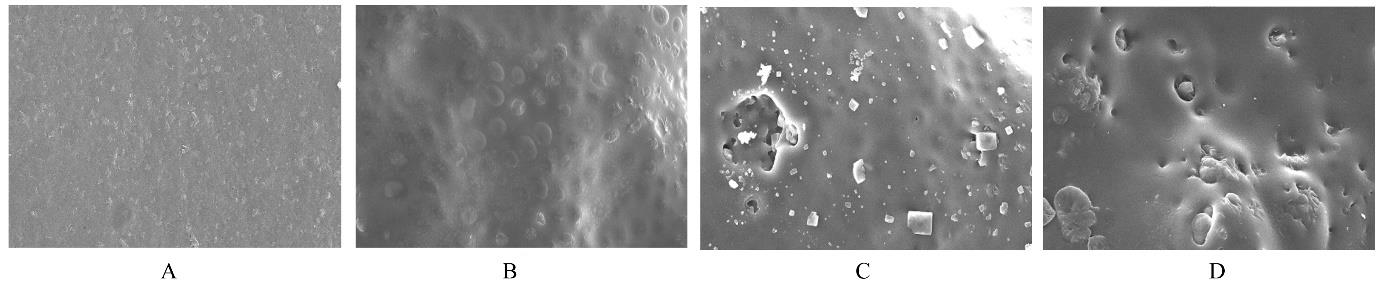
目的 制备与人体骨外膜结构相仿的聚碳酸1,2-丙二酯(PPC)/聚丁二酸丁二醇酯(PBS)生物膜,并评价其理化性能和生物学特征。 方法 采用盐析法制备PPC/PBS生物膜,核磁共振氢谱(1HRNM)观察PPC、PBS和 PPC/PBS的谱吸收峰,分析其共混前后化学结构变化。扫描电子显微镜(SEM)观察PPC/PBS生物膜超微形态表现,GPC凝膜渗透色谱仪检测其孔隙率、杨氏模量、断裂强度、断裂伸长率和静态接触角等理化性能。分离、培养和纯化原代SD大鼠成骨细胞,分为对照组(未加生物膜)、BME-10X胶原膜组和PPC/PBS生物膜组,SEM观察各组成骨细胞附着和生长情况,细胞计数法检测各组成骨细胞数量,碱性磷酸酶(ALP)试剂盒检测各组成骨细胞分化情况,兔背部肌肉降解实验检测PPC/PBS生物膜体内降解性能。 结果 PPC/PBS生物膜具有光滑面层和粗糙面层双层样结构,全层厚约为0.5 mm,平均孔径约为120 μm;孔隙率约为77.4%;杨氏模量约为38.1 MPa,断裂强度约为1.22 MPa,断裂伸长率约为7.4%;粗糙面接触角为85°,光滑面接触角为57°。SEM观察,培养1 d时,PPC/PBS生物膜表面附着的细胞较少;培养3、7和14 d时,可见大量成骨细胞附着于生物膜表面生长,细胞伸出伪足附着于生物膜上,胞体可呈悬空样分布于孔隙中。与对照组比较,培养1、3、7和14 d时,BME-10X胶原膜组和PPC/PBS复合膜组材料表面附着成骨细胞数量均减少(P<0.05)。与BME-10X胶原膜组比较,培养1、3、7和14 d时,PPC/PBS生物膜组材料表面附着成骨细胞数量均增加(P<0.05)。与对照组比较,培养1、3、7和14 d时,BME-10X胶原膜组和PPC/PBS生物膜组材料表面附着成骨细胞中ALP水平明显减少(P<0.01)。与BME-10X胶原膜组比较,培养1和3 d时,PPC/PBS生物膜组材料表面附着成骨细胞中ALP水平明显增加(P<0.01)。降解实验中,与降解0 周时比较,降解4 周时,PPC/PBS生物膜失重率和相对分子质量失重率均升高(P<0.05),断裂强度和断裂伸长率均明显减小(P<0.05或P<0.01)。自降解第2周开始,PPC/PBS生物膜粗糙面表面微孔样结构数逐渐增多,孔径为0~10 μm;至降解26周时,PPC/PBS生物膜粗糙面表面微孔样结构均匀分布;降解4周时,PPC/PBS生物膜光滑面表面出现脱屑样改变,但未见有微孔样结构;降解12周时,光滑面表面出现少量微孔样改变,微孔直径<5 μm;降解26周时,光滑面表面微孔样结构数增加,孔径<10 μm。 结论 PPC/PBS生物膜与人体骨外膜结构相似,呈双层样结构,亲水性好,光滑面层表面较为致密,粗糙面层孔隙率高,生物相容性好,且降解缓慢,是一种理想的引导再生生物膜。
中图分类号:
- R783.1