吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (5): 1275-1285.doi: 10.13481/j.1671-587X.20240511
• 基础研究 • 上一篇
水泡性口炎病毒对体外血管内皮屏障功能的损伤作用及其机制
曹宇璇1,陈为2,3,孙成彪2,赵娜2,王燕2,董明鑫2,许娜4,刘文森2( ),李咏梅1(
),李咏梅1( )
)
- 1.北华大学基础医学院病原生物学教研室, 吉林 吉林 132013
2.中国农业科学院长春兽医研究所动物性食品安全与生物交叉研究室, 吉林 长春 130122
3.河南科技大学应用工程学院食品医药 教研组, 河南 三门峡 472099
4.吉林医药学院教务处, 吉林 吉林 132013
Damage effect of VSV on vascular endothelial barrier function in vitro and its mechanism
Yuxuan CAO1,Wei CHEN2,3,Chengbiao SUN2,Na ZHAO2,Yan WANG2,Mingxin DONG2,Na XU4,Wensen LIU2( ),Yongmei LI1(
),Yongmei LI1( )
)
- 1.Department of Pathogen Biology,School of Basic Medical Sciences,Beihua University,Jilin 132013,China
2.Animal Food Safety and Biological Intersection Research Laboratory,Changchun Institute of Veterinary Medicine,Chinese Agricultural Sciences,Changchun 130122,China
3.Food and Medicine Teaching and Research Group,School of Applied Engineering,Henan University of Science and Technology,Sanmenxia 472099,China
4.Academic Affairs Office,Jilin Medical University,Jilin 132013,China
摘要:
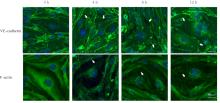
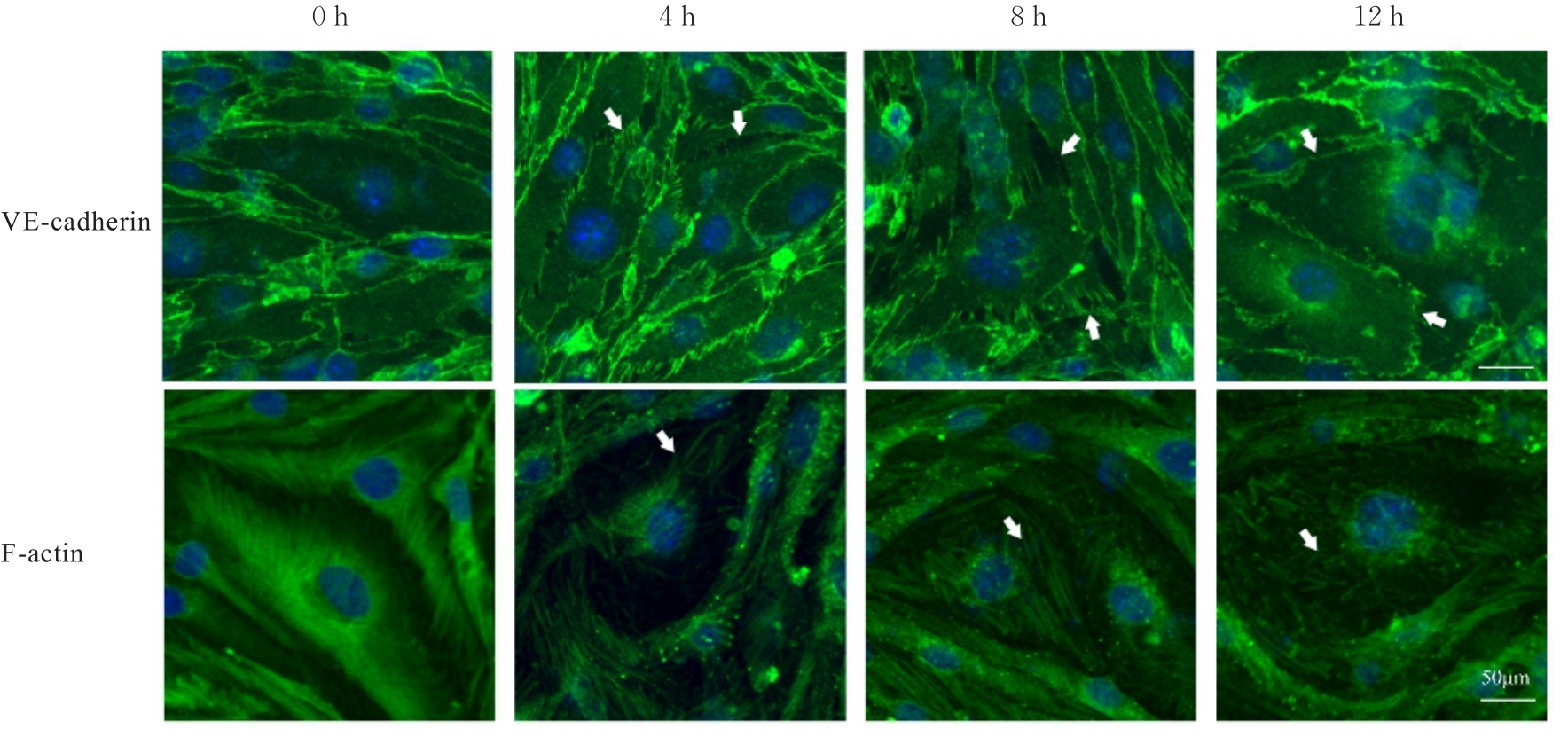
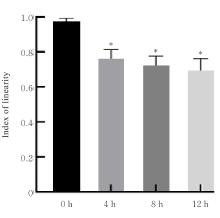
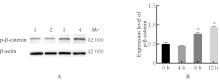
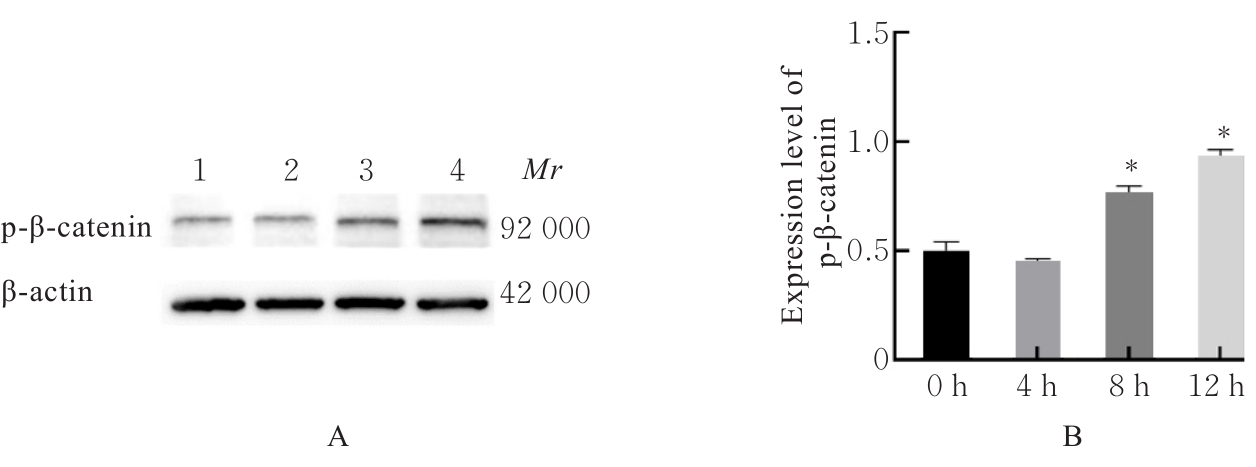
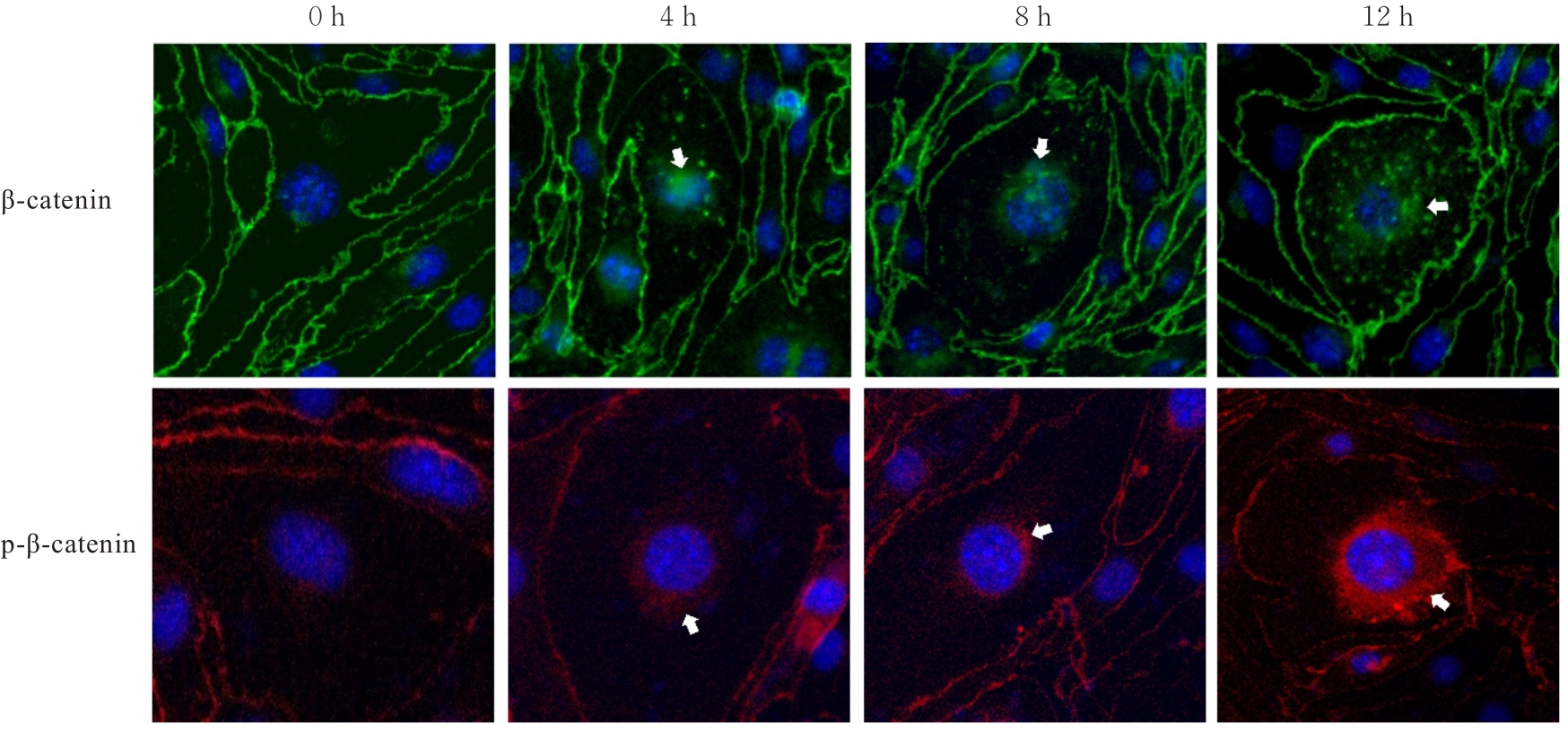
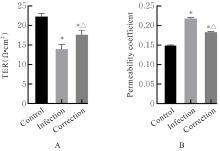
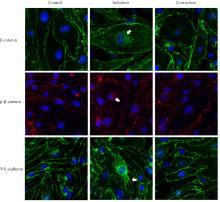
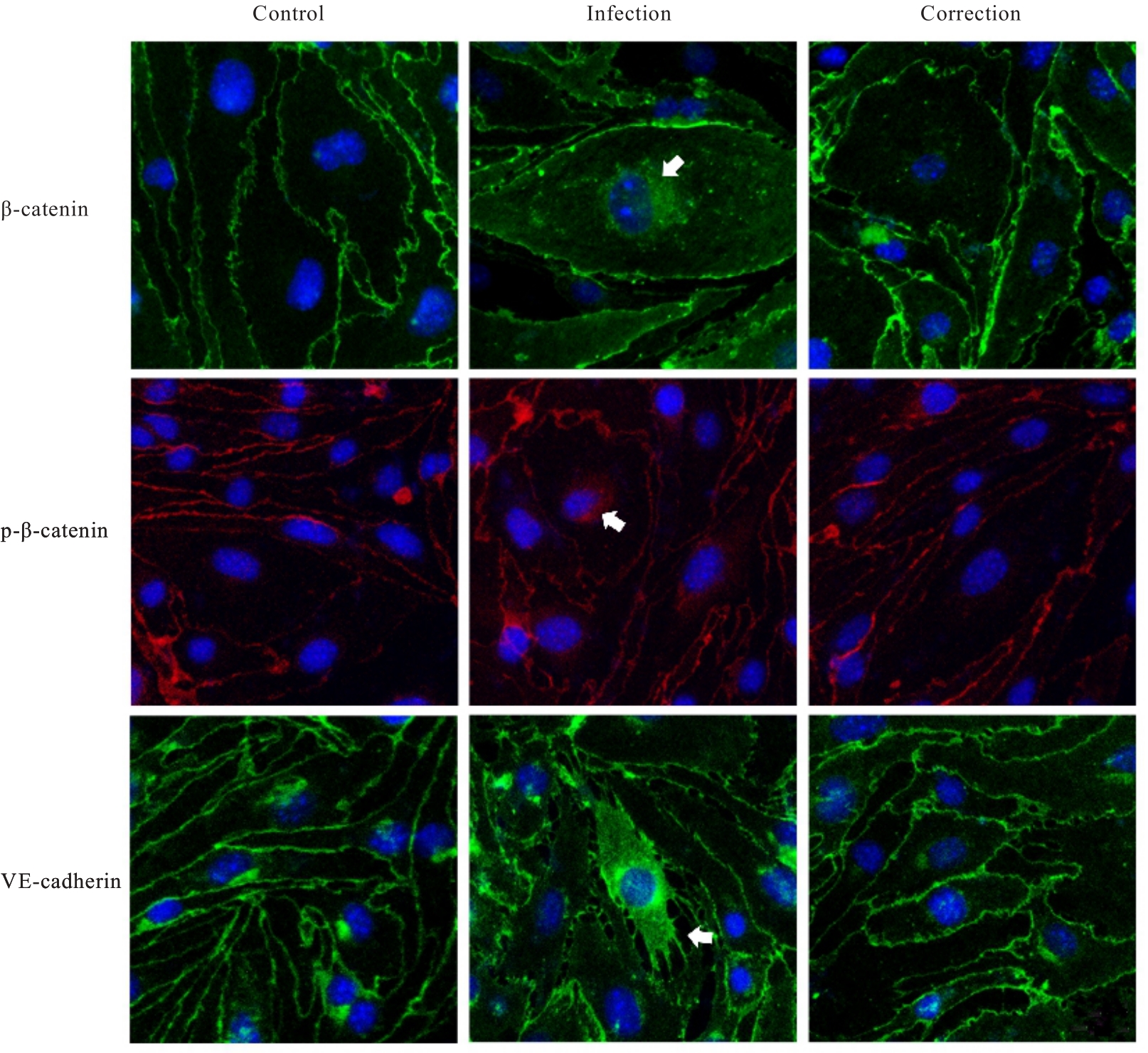
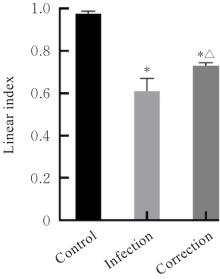
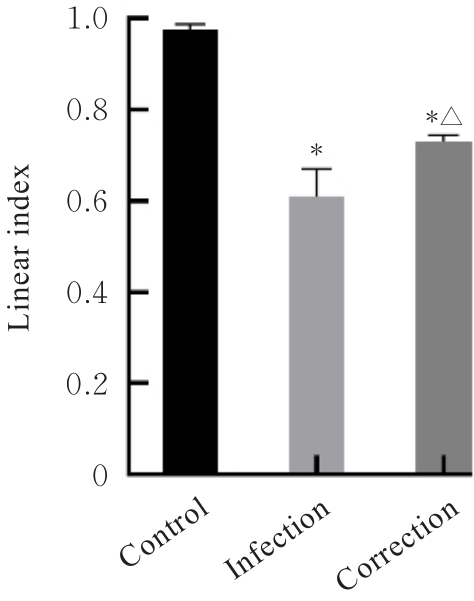
目的 探讨水泡性口炎病毒(VSV)对血管内皮(VE)屏障的损伤作用,并阐明其作用机制。 方法 采用犬肾细胞对VSV进行扩增,采用小鼠脑血管内皮瘤bEnd.3细胞检测VSV的半数组织培养感染剂量(TCID50),采用300倍TCID50进行后续实验。将bEnd.3细胞分为感染0 h组、感染4 h组、感染8 h组和感染12 h组,进行VSV感染损伤VE屏障实验;将bEnd.3细胞分为对照组、感染组和纠正组,进行抑制VSV复制与VE屏障恢复实验。将bEnd.3细胞接种于Transwell小室,构建体外VE屏障模型,采用细胞电压电阻仪检测感染VSV不同时间后各组bEnd.3细胞中跨上皮电阻(TER),采用异硫氰酸荧光素-葡聚糖渗漏实验检测各组渗透系数,采用免疫荧光染色法观察VSV感染后各组bEnd.3细胞骨架及黏附连接(AJs)中VE-钙黏蛋白、β-连环蛋白(β-catenin)和磷酸化β-连环蛋白(p-β-catenin)定位变化,采用实时荧光定量PCR(RT-qPCR)法检测各组细胞中Wnt和β-catenin mRNA表达水平,采用Western blotting法检测各组细胞中Wnt、β-catenin和p-β-catenin表达水平。 结果 VSV的TCID50为10-4.5·100 μL-1。Transwell小室实验检测,与感染0 h比较,其他各组细胞中TER明显降低(P<0.05),渗透系数明显升高(P<0.05)。免疫荧光染色,与对照组比较,感染组bEnd.3细胞骨架紊乱,细胞间隙增大,AJs线性指数明显降低(P<0.05),β-catenin和p-β-catenin从细胞膜转移至细胞核周围。RT-qPCR法检测,与感染0 h比较,其他各组细胞中Wnt mRNA表达水平明显降低(P<0.05),β-catenin mRNA表达水平差异无统计学意义(P>0.05)。Western blotting法检测,与感染0 h比较,其他各组细胞中Wnt蛋白表达水平明显降低(P<0.05),β-catenin表达水平差异无统计学意义(P>0.05),p-β-catenin表达水平明显升高(P<0.05)。在抑制VSV复制并纠正低密度脂蛋白受体(LDLR)异常后,Transwell小室实验检测,与感染组比较,纠正组 bEnd.3细胞中TER明显升高(P<0.05),渗透系数明显降低(P<0.05)。免疫荧光染色,与感染组比较,纠正组细胞间隙减小,细胞中β-catenin和p-β-catenin核周聚集现象有所改善。RT-qPCR法检测,与感染组比较,纠正组细胞中Wnt mRNA表达水平明显升高(P<0.05)。Western blotting法检测,与感染组比较,纠正组细胞中Wnt蛋白表达水平明显升高(P<0.05),β-catenin表达水平差异无统计学意义(P>0.05),p-β-catenin表达水平明显降低(P<0.05)。 结论 VSV感染后可引起LDLR失活,降低Wnt蛋白表达水平,造成β-catenin磷酸化水平升高并发生内化,破坏AJs稳定性,最终导致VE屏障损伤。
中图分类号:
- S852.65