吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (4): 900-907.doi: 10.13481/j.1671-587X.20240403
血管性血友病因子裂解酶间隔区结构域突变对酶生物学功能的影响
- 1.延边大学附属医院血液内科,吉林 延吉 133000
2.浙江省绍兴市人民医院康复医学科,浙江 绍兴 312000
3.延边大学附属医院体检科,吉林 延吉 133000
4.延边大学附属医院 中心实验室,吉林 延吉133000
Effect of ADAMTS13 spacer domain mutations on biological function of enzyme
Meng WANG1,Hao WU2,Hua LI3,Yihong ZHAO1,Shengyu JIN1,4( )
)
- 1.Department of Hematology, Affiliated Hospital, Yanbian University, Yanji 133000, China
2.Department of Rehabilitation, People’s Hospital, Shaoxing City, Zhejiang Province, Shaoxing 312000, China
3.Department of Physical Examination, Affiliated Hospital, Yanbian University, Yanji 133000, China
4.Central Laboratory, Affiliated Hospital, Yanbian University, Yanji 133000, China
摘要:
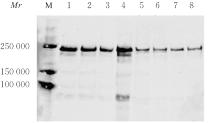
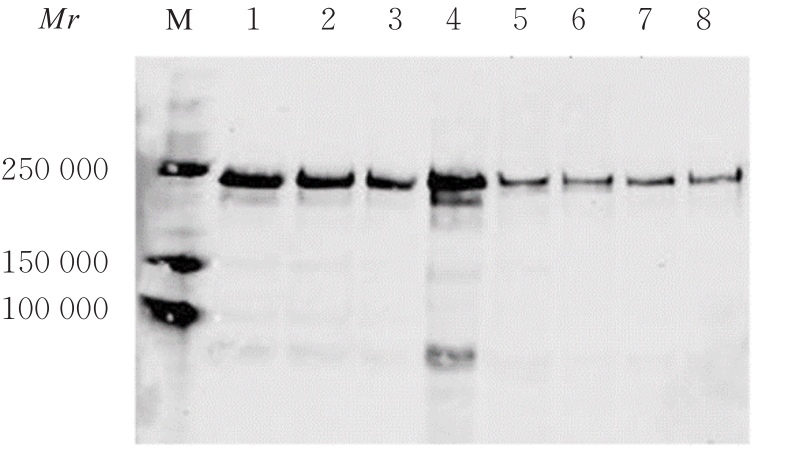
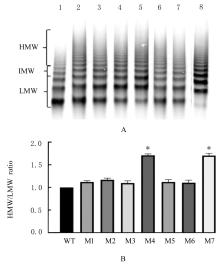
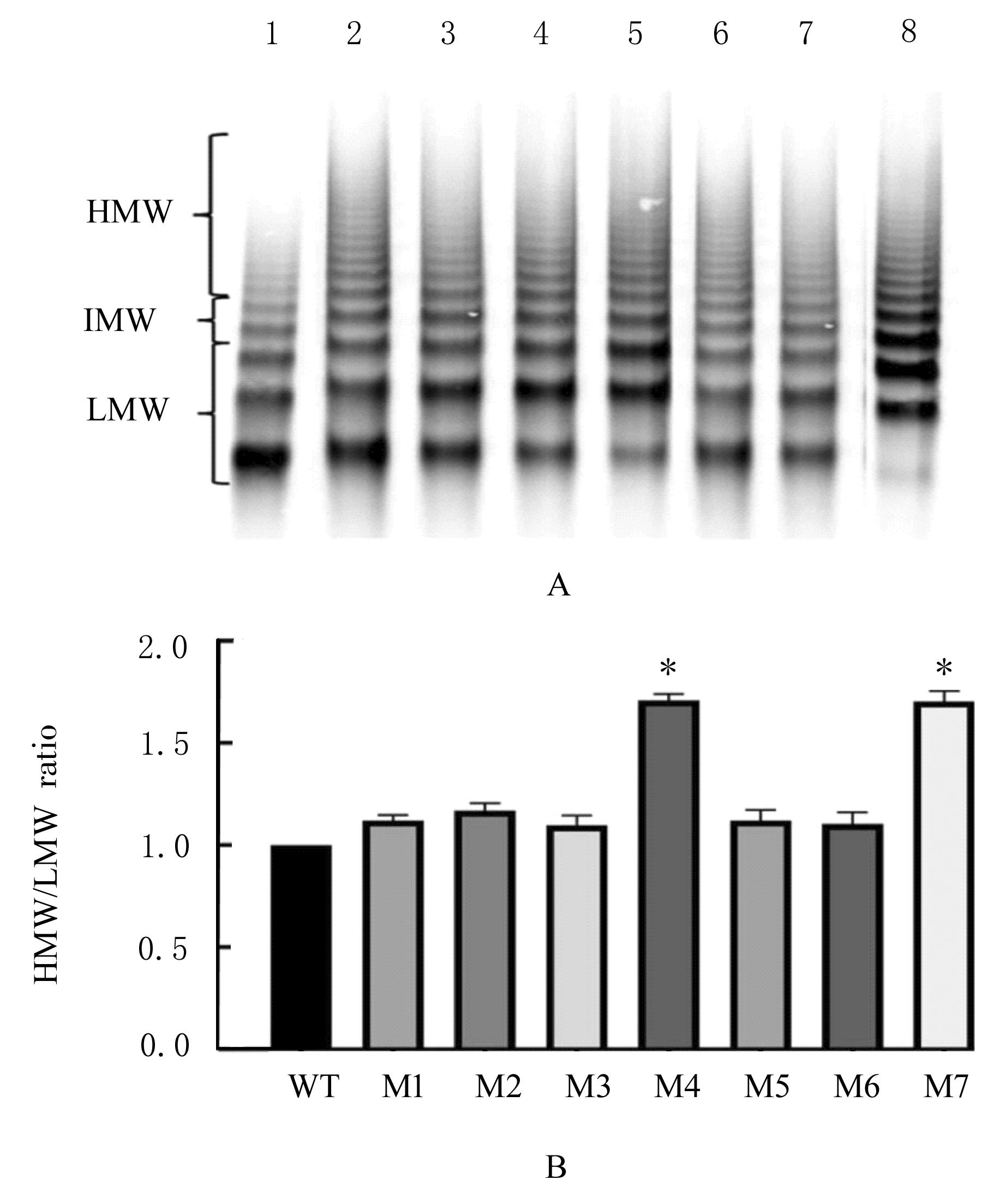
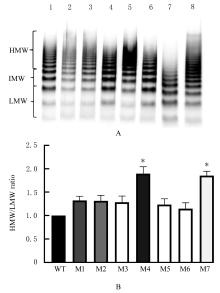
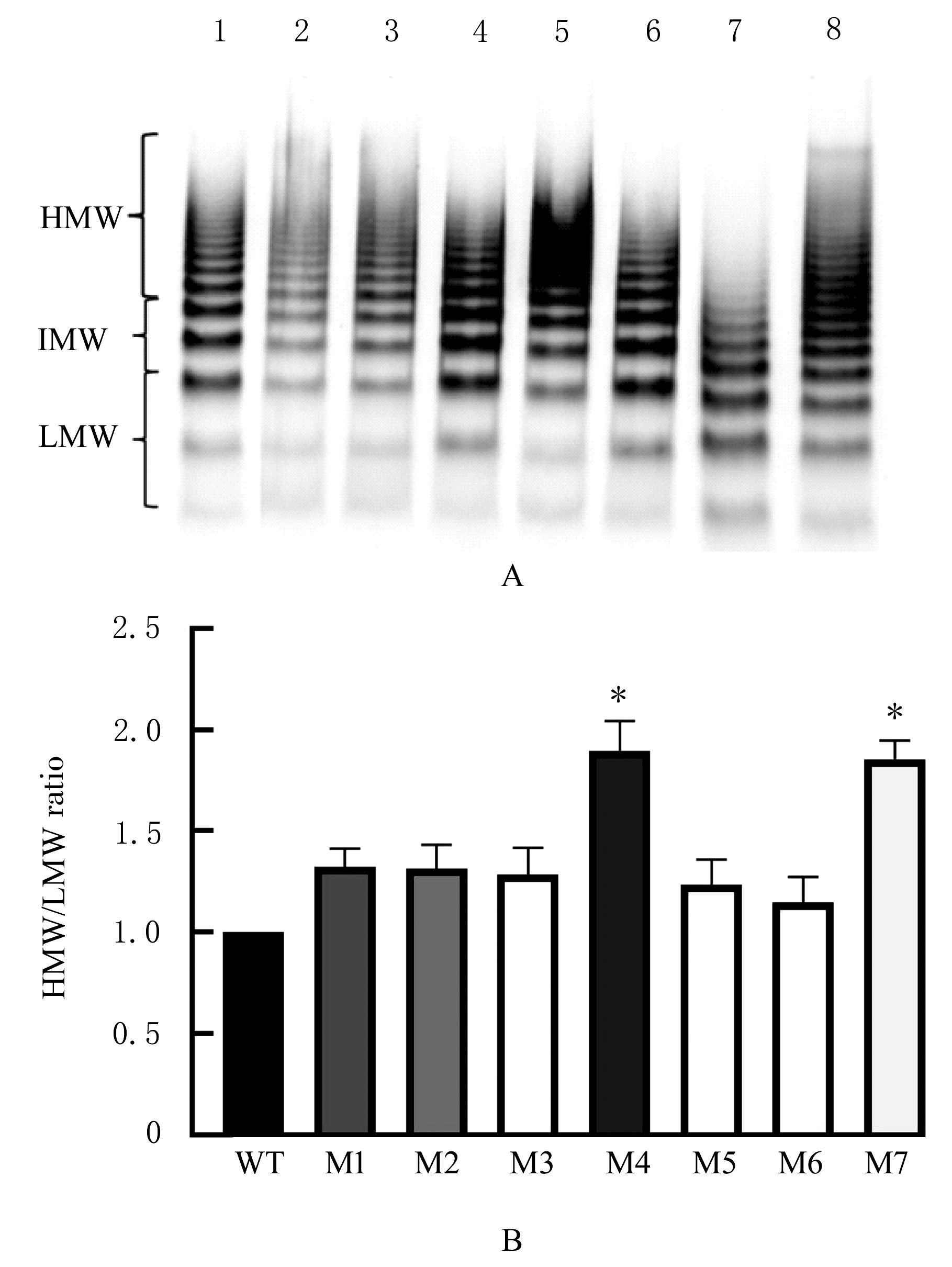
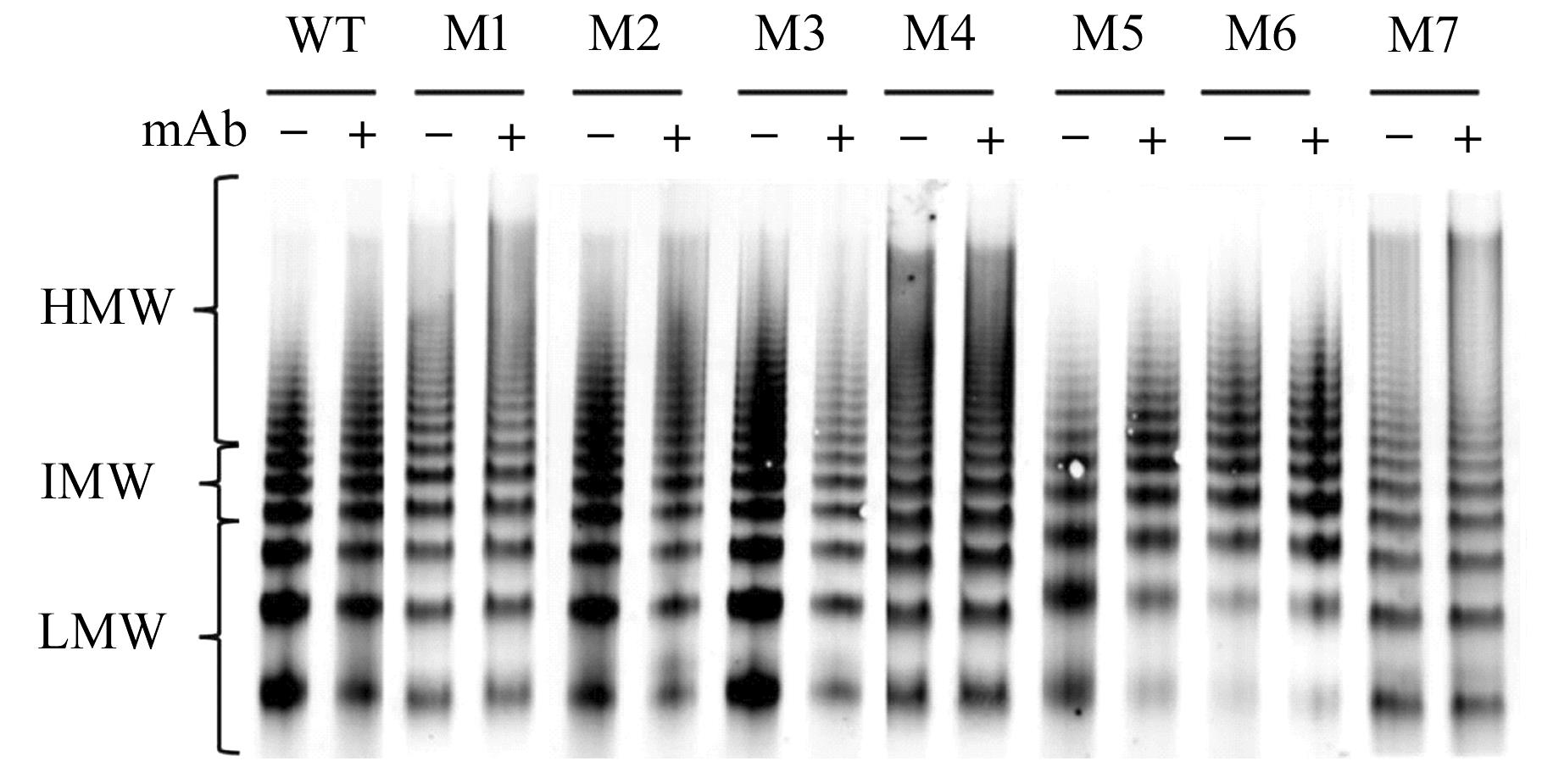
目的 探讨血管性血友病因子裂解酶 ADAM 金属肽酶含血小板反应蛋白1型13(ADAMTS13)间隔区结构域在血管性血友病因子(vWF)裂解过程中的生物学功能,阐明ADAMTS13在血栓性血小板减少性紫癜(TTP)发病机制中的作用。 方法 将ADAMTS13间隔区结构域中的氨基酸残基 TEDRLPR 以点突变技术逐个基因突变(突变体M1~M7),将构建的ADAMTS13 与其突变体质粒转染至人胚肾 HEK293 细胞,稳定表达后提纯重组蛋白。观察野生型和突变型 ADAMTS13 在变性条件、剪切应力作用和 ADAMTS13 抗体处理后裂解 vWF 的能力。 结果 荧光共振能量转移(FRET)实验, 与野生型 ADAMTS13 比较, ADAMTS13 突变体M4(R635A)和突变体M7(R638A)对FRET-vWF73剪切能力降低(P<0.05)。变性条件下,野生型ADAMTS13可以将vWF多聚体裂解, 与野生型ADAMTS13比较, ADAMTS13突变体M4(R635A)和突变体M7(R638A)的裂解活性明显降低(P<0.01)。在体外剪切应力作用下,与野生型 ADAMTS13 比较, ADAMTS13 突变体 M4(R635A)和突变体M7(R638A)裂解 vWF 多聚体的能力明显降低(P<0.01)。 与野生型 ADAMTS13 比较, ADAMTS13突变体M4(R635A)和突变体M7(R638A)与vWF之间的结合力差异无统计学意义(P>0.05),表明ADAMTS13的C末端与vWF之间存在多个结合位点。ADAMTS13抗体处理可在一定程度上抑制野生型和突变型ADAMTS13裂解vWF的能力。 结论 间隔区突变后ADAMTS13的活性降低。ADAMTS13突变体M4(R635A)和突变体M7(R638A)可能是ADAMTS13在底物识别时的重要作用位点。
中图分类号:
- R554.6