吉林大学学报(医学版) ›› 2021, Vol. 47 ›› Issue (3): 770-776.doi: 10.13481/j.1671-587X.20210330
检测β⁃CTX和N⁃MID水平的双标记时间分辨免疫荧光分析方法的建立和评价
毛骞1,陈翠翠2,梁焕坤2,刘鹏娥2,钟树海2,李来庆2( )
)
- 1.北华大学附属医院内分泌科,吉林 吉林 132001
2.广州优迪生物科技股份有限公司,广东 广州 510663
Establishment and evaluation of a double⁃labeled time⁃ resolved immunofluorescence analysis method for detecting levels of β⁃CTX and N⁃MID
Qian MAO1,Cuicui CHEN2,Huankun LIANG2,Penge LIU2,Shuhai ZHONG2,Laiqing LI2( )
)
- 1.Department of Endocrinology,Affiliated Hospital,Beihua University,Jilin 132001,China
2.Guangzhou Youdi Biotechnology Co. ,Ltd. ,Guangzhou 510663,China
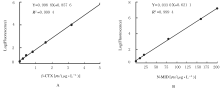
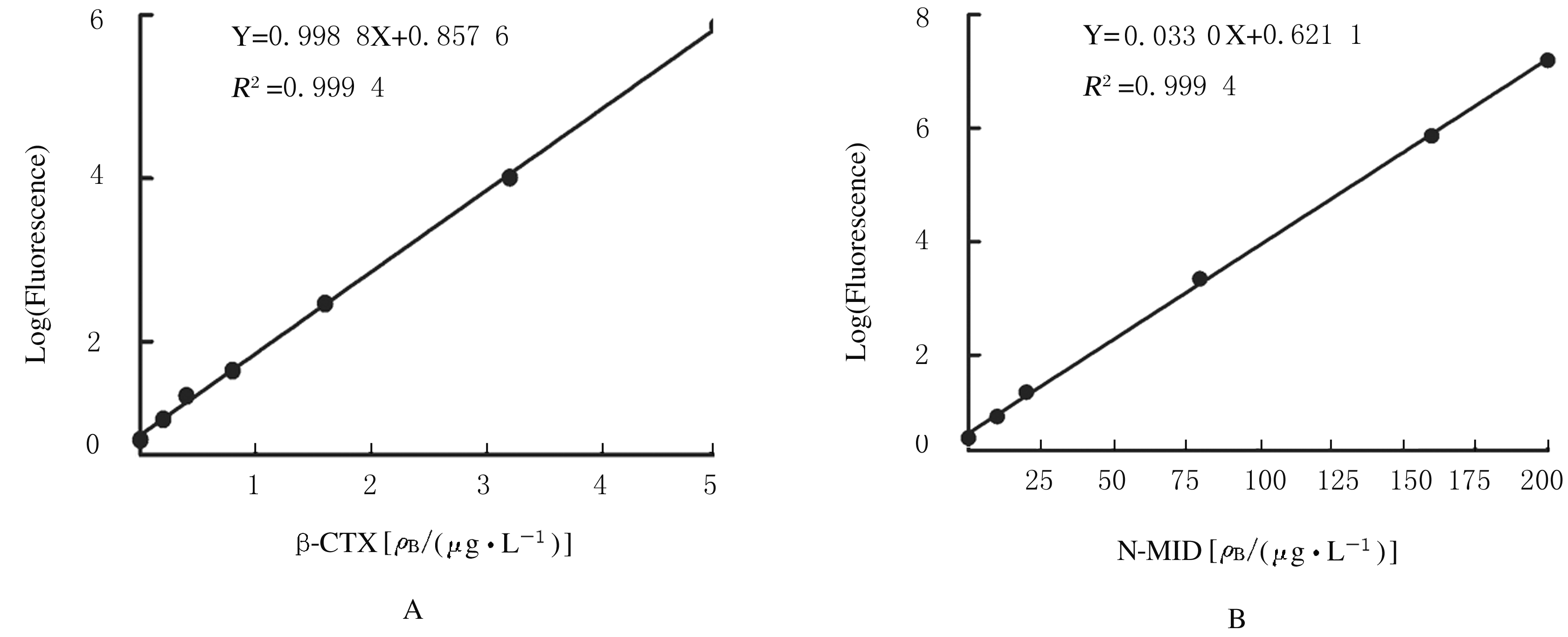
摘要: 研制一种检测β胶联降解产物(β-CTX)和骨钙素N端中分子片段(N-MID)水平的双标记时间分辨免疫荧光分析方法(TRFIA)并评价其检测性能。 将抗β-CTX和N-MID的4E5和2B7单克隆抗体(MAb)作为包被抗体包被在96孔培养板,采用铕离子(Eu3+)和钐离子(Sm3+)分别标记2G6和5A3 MAb作为检测抗体,建立双抗体夹心TRFIA分析法并制备成试剂盒。通过试剂盒的灵敏度、准确度(稀释回收率)、特异性、精密度、稳定性和临床样本比对等实验评价其检测性能。 制备的双标记TRFIA试剂盒对β-CTX的检测灵敏度为0.025 μg·L-1,线性范围为0.025 ~ 5.000 μg·L-1,对N-MID的检测灵敏度为0.5 μg·L-1,线性范围为0.5~200.0 μg·L-1。β-CTX平均稀释回收率为102.13%,N-MID平均稀释回收率为103.02%,与其他常见骨检测指标无明显交叉反应,特异性较强。β-CTX 批内变异系数(CV)为5.81%~7.82%,批间CV为5.97%~8.02%;N-MID批内CV 为6.05%~8.32%,批间CV为6.14%~8.56%;TRFIA试剂盒可在4 ℃稳定保存6个月,37 ℃稳定保存7 d。 建立的双标记TRFIA方法具有高灵敏度、高特异度、高准确率和方便快捷等优点,适用于大批量临床样品的检测。
中图分类号:
- R331