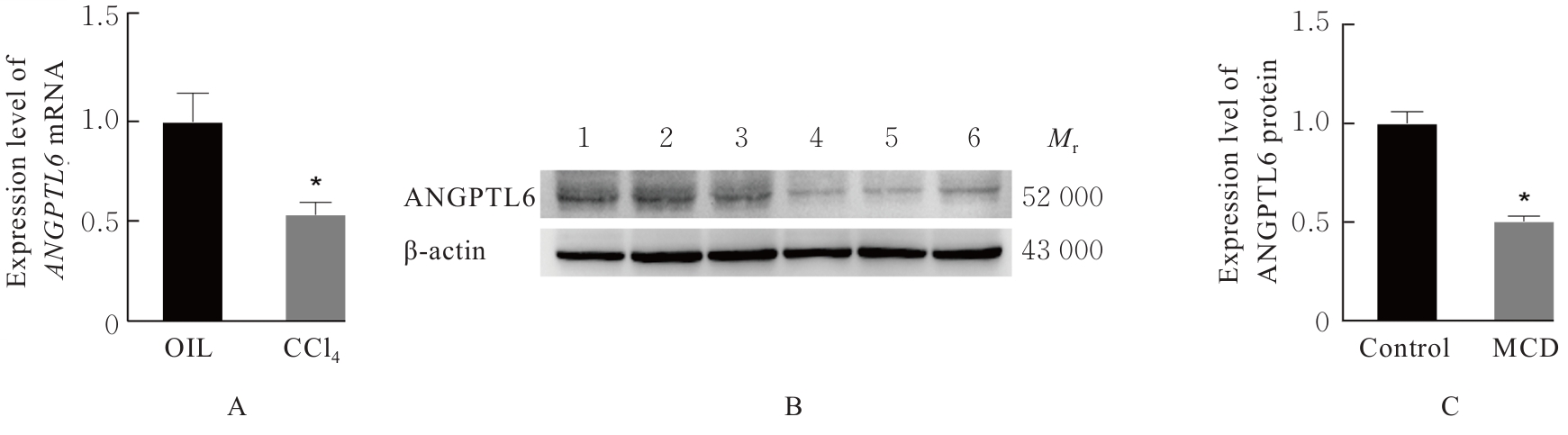
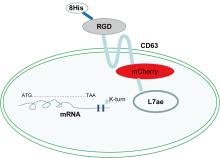
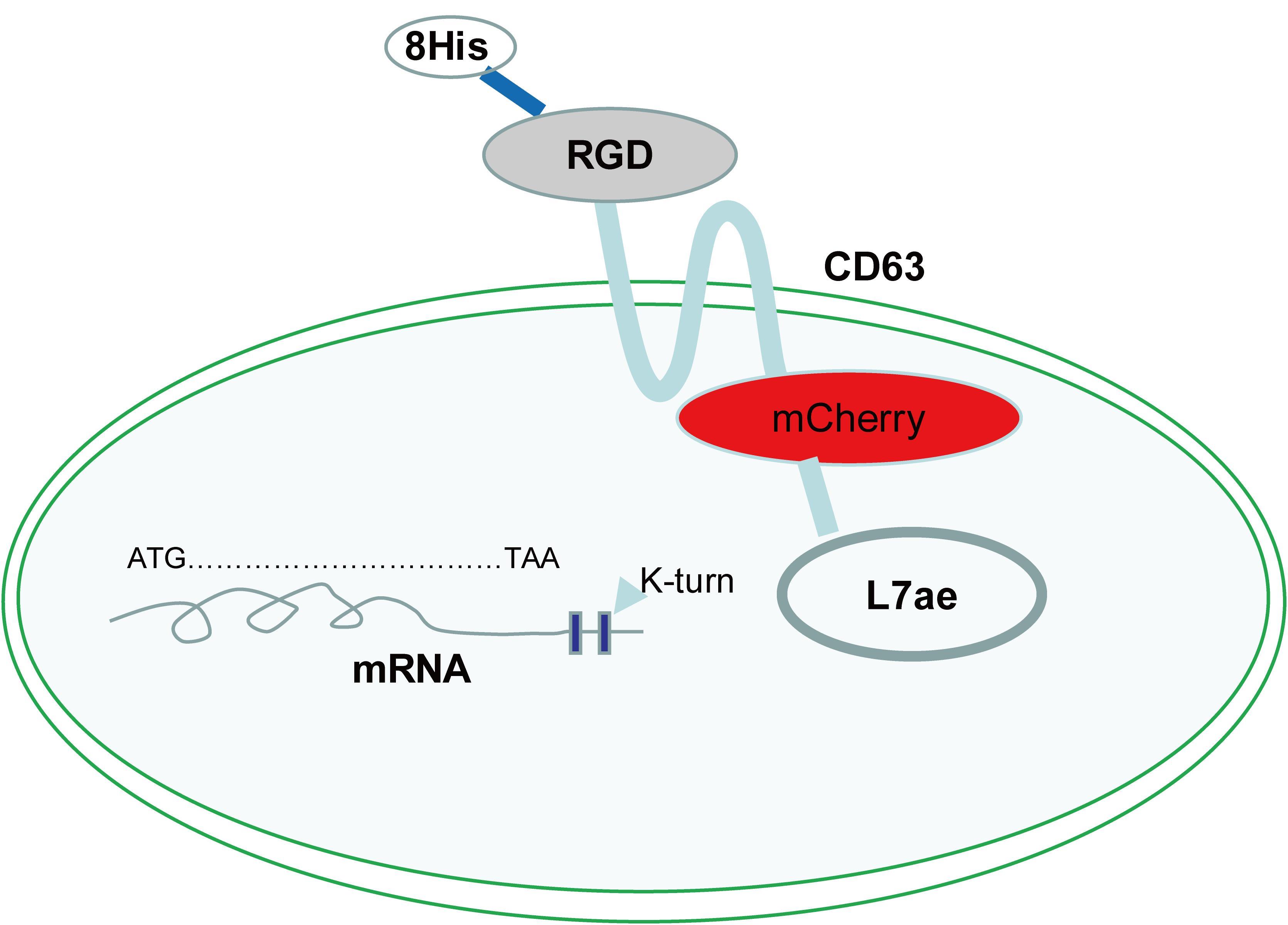
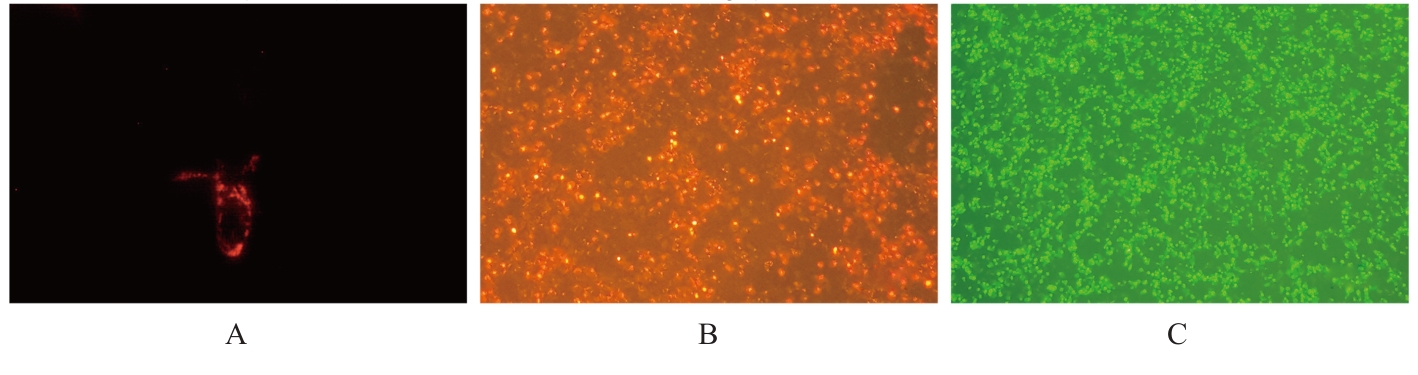
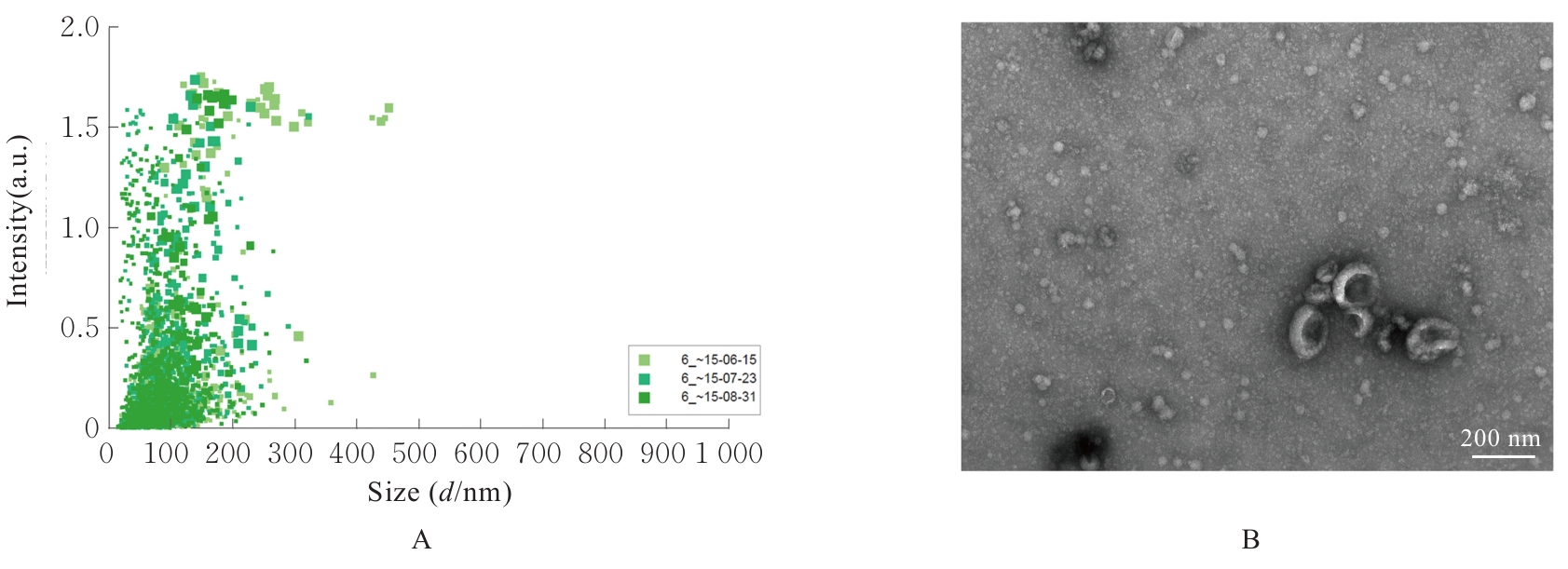
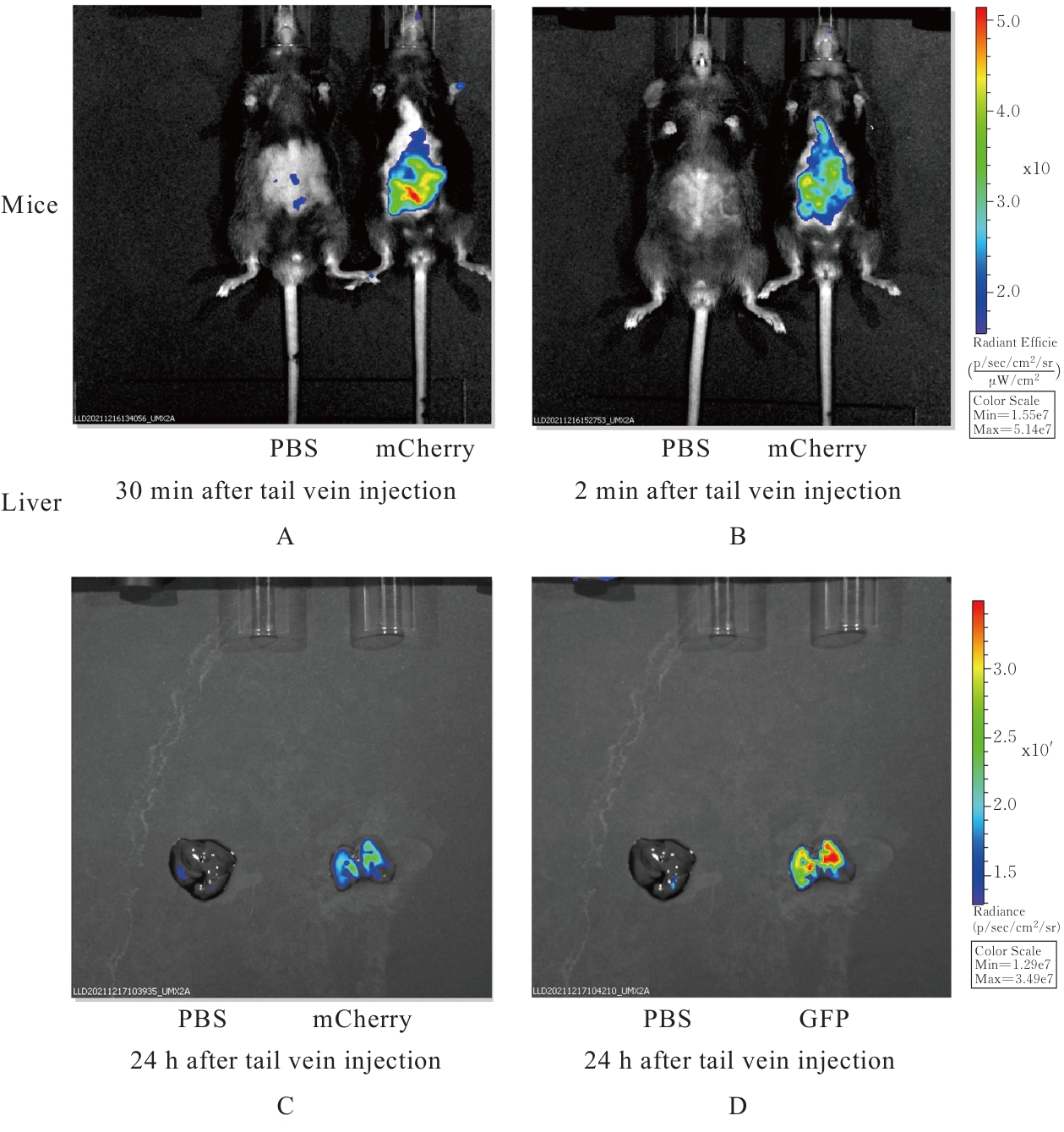
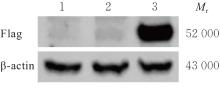
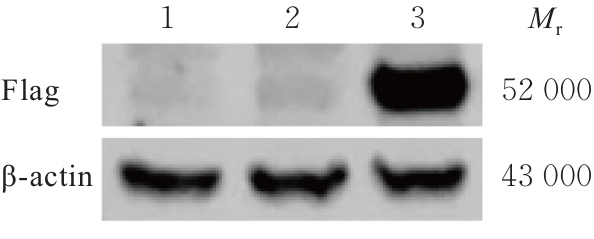
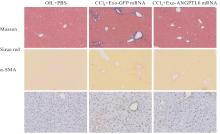
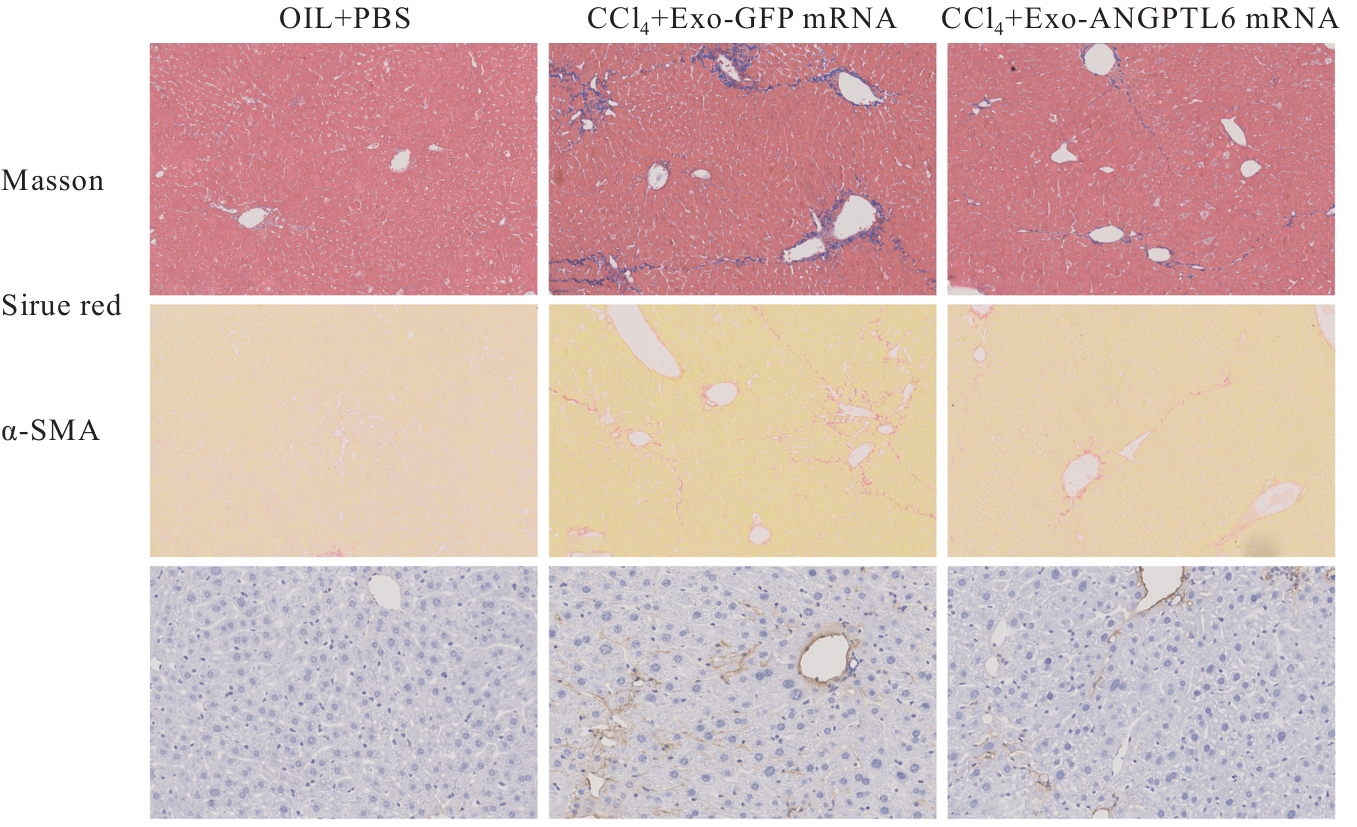
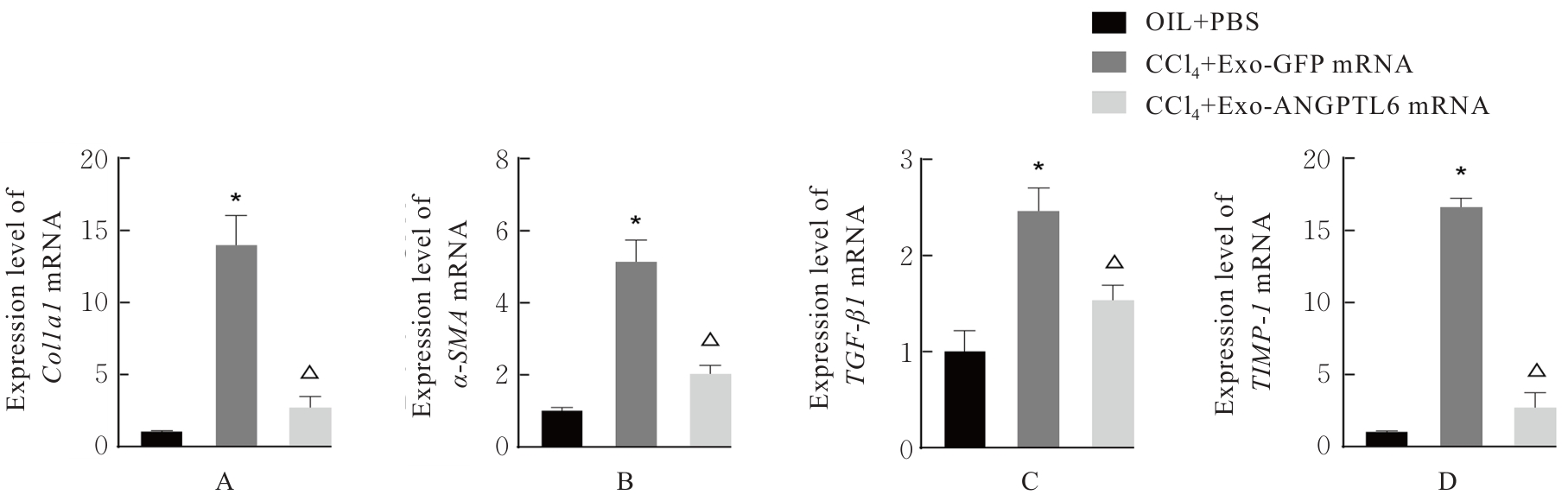
| [1] |
ROEHLEN N, CROUCHET E, BAUMERT T F. Liver fibrosis: mechanistic concepts and therapeutic perspectives[J]. Cells, 2020, 9(4): 875.
|
| [2] |
PAROLA M, PINZANI M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues[J]. Mol Aspects Med, 2019, 65: 37-55.
|
| [3] |
WANG Y K, WANG M Q, LIU C R, et al. Global burden of liver cirrhosis 1990-2019 and 20 years forecast: results from the global burden of disease study 2019[J]. Ann Med, 2024, 56(1): 2328521.
|
| [4] |
MOHAMMED O S, ATTIA H G, MOHAMED B M S A, et al. Current investigations for liver fibrosis treatment: between repurposing the FDA-approved drugs and the other emerging approaches[J]. J Pharm Pharm Sci, 2023, 26: 11808.
|
| [5] |
ZHANG C Y, LIU S, YANG M. Treatment of liver fibrosis: Past, current, and future[J]. World J Hepatol, 2023, 15(6): 755-774.
|
| [6] |
OIKE Y, YASUNAGA K, ITO Y, et al. Angiopoietin-related growth factor (AGF) promotes epidermal proliferation, remodeling, and regeneration[J]. Proc Natl Acad Sci U S A, 2003, 100(16): 9494-9499.
|
| [7] |
OIKE Y, AKAO M, YASUNAGA K, et al. Angiopoietin-related growth factor antagonizes obesity and insulin resistance[J]. Nat Med, 2005, 11(4): 400-408.
|
| [8] |
HOSTETTLER I C, O’CALLAGHAN B, BUGIARDINI E, et al. ANGPTL6 genetic variants are an underlying cause of familial intracranial aneurysms[J]. Neurology, 2021, 96(6): e947-e955.
|
| [9] |
WANG Z H, TANG M Z, RONG Y, et al. ANGPTL6 can be used as potential biomarkers for the diagnosis and prognosis of thyroid cancer[J]. Asian J Surg, 2024, 47(7): 3079-3081.
|
| [10] |
QADDOUMI M G, ALANBAEI M, HAMMAD M M, et al. Investigating the role of myeloperoxidase and angiopoietin-like protein 6 in obesity and diabetes[J]. Sci Rep, 2020, 10(1): 6170.
|
| [11] |
ABELS E R, BREAKEFIELD X O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake[J]. Cell Mol Neurobiol, 2016, 36(3): 301-312.
|
| [12] |
RAFIEEZADEH D. Extracellular vesicles and their therapeutic applications: a review article (part 2)[J]. Int J Physiol Pathophysiol Pharmacol, 2024, 16(4): 81-88.
|
| [13] |
FAN W G, LIU T H, CHEN W, et al. ECM1 prevents activation of transforming growth factor β, hepatic stellate cells, and fibrogenesis in mice[J]. Gastroenterology, 2019, 157(5): 1352-1367.e13.
|
| [14] |
HORN P, TACKE F. Metabolic reprogramming in liver fibrosis[J]. Cell Metab, 2024, 36(7): 1439-1455.
|
| [15] |
KAMM D R, MCCOMMIS K S. Hepatic stellate cells in physiology and pathology[J]. J Physiol, 2022, 600(8): 1825-1837.
|
| [16] |
ZHANG M F, SERNA-SALAS S, DAMBA T, et al. Hepatic stellate cell senescence in liver fibrosis: Characteristics, mechanisms and perspectives[J]. Mech Ageing Dev, 2021, 199: 111572.
|
| [17] |
KONG M, ZHOU J J, KANG A Q, et al. Histone methyltransferase Suv39h1 regulates hepatic stellate cell activation and is targetable in liver fibrosis[J]. Gut, 2024, 73(5): 810-824.
|
| [18] |
DING J, XU C, XU M, et al. Emerging role of engineered exosomes in nonalcoholic fatty liver disease[J]. World J Hepatol, 2023, 15(3): 386-392.
|
| [19] |
KOJIMA R, BOJAR D, RIZZI G, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment[J]. Nat Commun, 2018, 9(1): 1305.
|
| [20] |
SAITO H, KOBAYASHI T, HARA T, et al. Synthetic translational regulation by an L7Ae-kink-turn RNP switch[J]. Nat Chem Biol, 2010, 6(1): 71-78.
|
| [21] |
LIANG Y J, DUAN L, LU J P, et al. Engineering exosomes for targeted drug delivery[J]. Theranostics, 2021, 11(7): 3183-3195.
|
| [22] |
URABE F, KOSAKA N, ITO K, et al. Extracellular vesicles as biomarkers and therapeutic targets for cancer[J]. Am J Physiol Cell Physiol, 2020, 318(1): C29-C39.
|
| [23] |
AMERNIA B, MOOSAVY S H, BANOOKH F, et al. FIB-4, APRI, and AST/ALT ratio compared to FibroScan for the assessment of hepatic fibrosis in patients with non-alcoholic fatty liver disease in Bandar Abbas, Iran[J]. BMC Gastroenterol, 2021, 21(1): 453.
|
| [24] |
DUAN B W, LIU Y J, LI X N, et al. An autologous macrophage-based phenotypic transformation-collagen degradation system treating advanced liver fibrosis[J]. Adv Sci, 2024, 11(7): 2306899.
|
| [25] |
XUE X Y, ZHAO X T, WANG J, et al. Carthami flos extract against carbon tetrachloride-induced liver fibrosis via alleviating angiogenesis in mice[J]. Phytomedicine, 2023, 108: 154517.
|
| [26] |
XU S X, CHEN Y E, MIAO J D, et al. Esculin inhibits hepatic stellate cell activation and CCl(4)-induced liver fibrosis by activating the Nrf2/GPX4 signaling pathway[J]. Phytomedicine, 2024, 128: 155465.
|
| [27] |
WANG C, ZHANG S L, LI Y Z, et al. Phillygenin inhibits TGF-β1-induced hepatic stellate cell activation and inflammation: regulation of the bax/bcl-2 and Wnt/β-catenin pathways[J]. Inflammation, 2024, 47(4): 1403-1422.
|
| [28] |
MEDEIROS T, SARAIVA G N, MORAES L A, et al. Liver fibrosis improvement in chronic hepatitis C after direct acting-antivirals is accompanied by reduced profibrogenic biomarkers-a role for MMP-9/TIMP-1[J]. Dig Liver Dis, 2020, 52(10): 1170-1177.
|
| [29] |
JI Y, DUAN Y L, LI Y Y, et al. A long-acting FGF21 attenuates metabolic dysfunction-associated steatohepatitis-related fibrosis by modulating NR4A1-mediated Ly6C phenotypic switch in macrophages[J]. Br J Pharmacol, 2024, 181(16): 2923-2946.
|
| [30] |
YANG J, CHEN L, ZHAO S S, et al. FGF21-dependent alleviation of cholestasis-induced liver fibrosis by sodium butyrate[J]. Front Pharmacol, 2024, 15: 1422770.
|
| [31] |
KANG S G, YI H S, CHOI M J, et al. ANGPTL6 expression is coupled with mitochondrial OXPHOS function to regulate adipose FGF21[J]. J Endocrinol, 2017, 233(1): 105-118.
|
 )
)