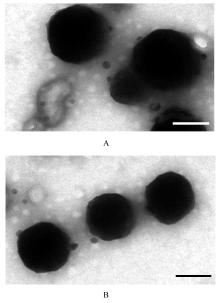
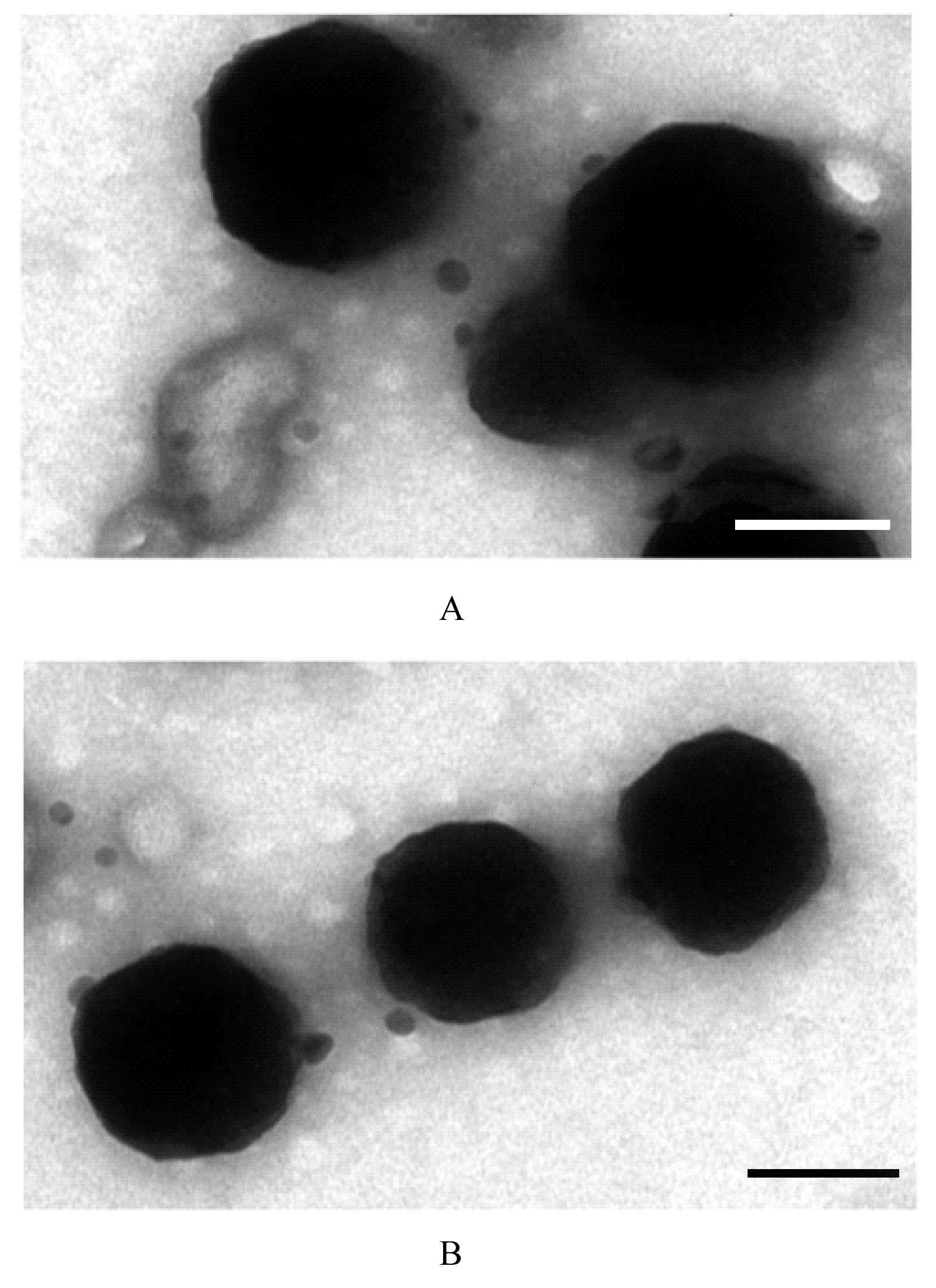
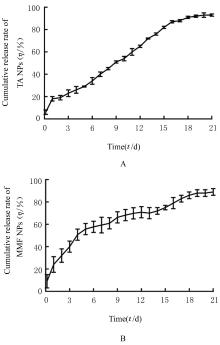
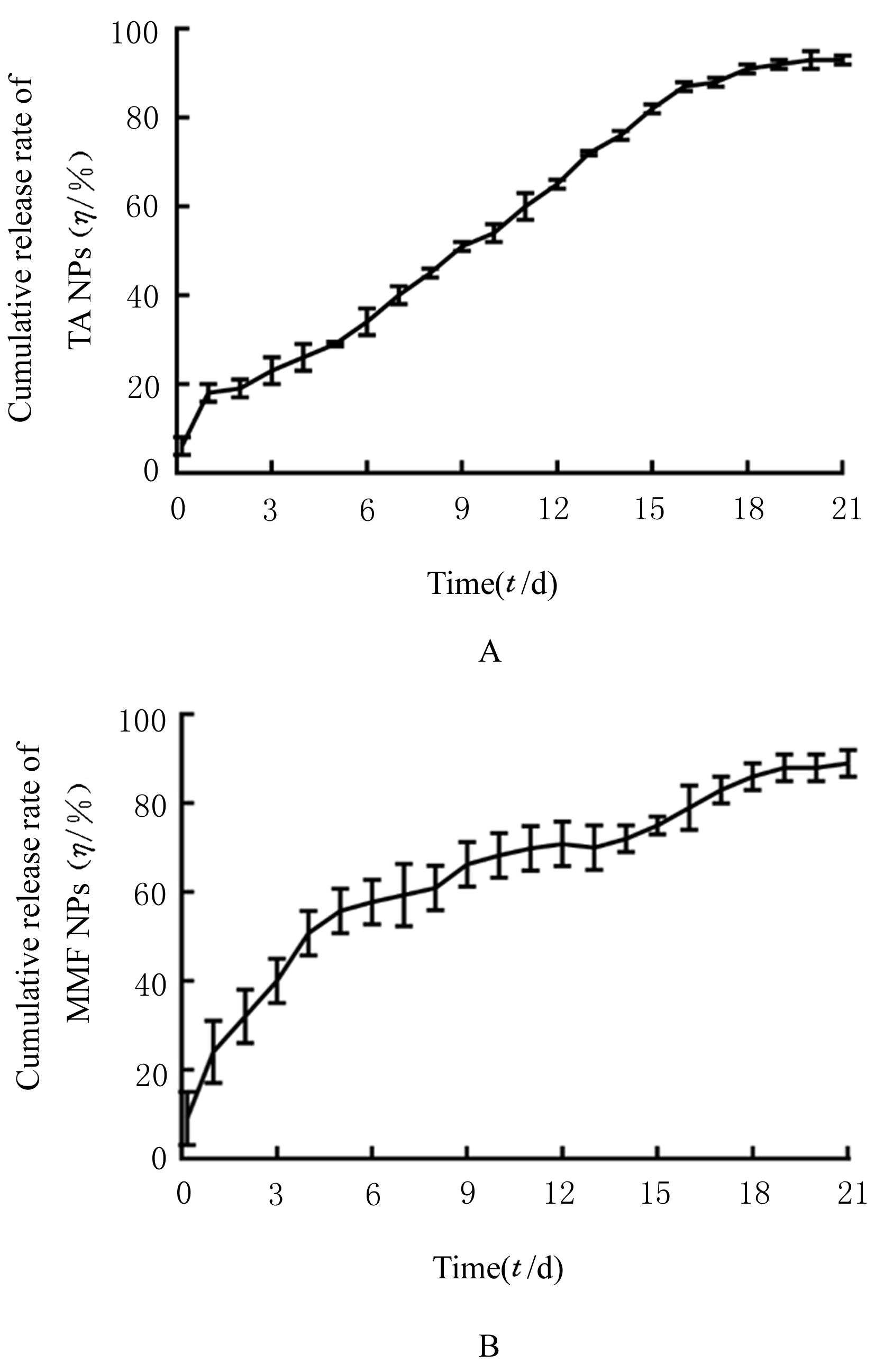
| 1 |
王 蕾, 马建民. 甲状腺相关眼病发病机制的研究进展[J]. 中华眼科杂志, 2017, 53(6):474-480.
|
| 2 |
SMITH T J, HEGEDÜS L. Graves’ disease[J]. N Engl J Med, 2016, 375(16): 1552-1565.
|
| 3 |
TURCK N, EPERON S, DE LOS ANGELES GRACIA M, et al. Thyroid-associated orbitopathy and biomarkers: where we are and what we can hope for the future[J]. Dis Markers, 2018, 2018: 7010196.
|
| 4 |
BARTALENA L, PIANTANIDA E, GALLO D,et al. Epidemiology, natural history, risk factors, and prevention of Graves’ orbitopathy[J]. Front Endocrinol, 2020, 11: 615993.
|
| 5 |
LEE M H, CHIN Y H, NG C H, et al. Risk factors of thyroid eye disease[J]. Endocr Pract, 2021, 27(3): 245-253.
|
| 6 |
NEAG E J, SMITH T J. 2021 update on thyroid-associated ophthalmopathy[J]. J Endocrinol Invest, 2022, 45(2): 235-259.
|
| 7 |
BARTALENA L, KAHALY G J, BALDESCHI L, et al. The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy[J]. Eur J Endocrinol, 2021, 185(4): G43-G67.
|
| 8 |
MOLETI M, GIUFFRIDA G, STURNIOLO G,et al. Acute liver damage following intravenous glucocorticoid treatment for Graves’ ophthalmopathy[J]. Endocrine, 2016, 54(1): 259-268.
|
| 9 |
MIŚKIEWICZ P, JANKOWSKA A, BRODZIŃSKA K,et al. Influence of methylprednisolone pulse therapy on liver function in patients with Graves’ orbitopathy[J]. Int J Endocrinol, 2018, 2018: 1978590.
|
| 10 |
MARCOCCI C, WATT T, ALTEA M A, et al. Fatal and non-fatal adverse events of glucocorticoid therapy for Graves’ orbitopathy: a questionnaire survey among members of the European Thyroid Association[J]. Eur J Endocrinol, 2012, 166(2): 247-253.
|
| 11 |
BARTALENA L, KRASSAS G E, WIERSINGA W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy[J]. J Clin Endocrinol Metab, 2012, 97(12): 4454-4463.
|
| 12 |
SALVI M, VANNUCCHI G, CURRÒ N, et al. Efficacy of B-cell targeted therapy with rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study[J]. J Clin Endocrinol Metab, 2015, 100(2): 422-431.
|
| 13 |
JANAGAM D R, WU L F, LOWE T L. Nanoparticles for drug delivery to the anterior segment of the eye[J]. Adv Drug Deliv Rev, 2017, 122: 31-64.
|
| 14 |
SWETLEDGE S, JUNG J P, CARTER R, et al. Distribution of polymeric nanoparticles in the eye: implications in ocular disease therapy[J]. J Nanobiotechnology, 2021, 19(1): 10.
|
| 15 |
MEZA-RIOS A, NAVARRO-PARTIDA J, ARMENDARIZ-BORUNDA J, et al. Therapies based on nanoparticles for eye drug delivery[J]. Ophthalmol Ther, 2020, 9(3): 1-14.
|
| 16 |
BEHAR-COHEN F. Recent advances in slow and sustained drug release for retina drug delivery[J]. Expert Opin Drug Deliv, 2019, 16(7): 679-686.
|
| 17 |
DUBASHYNSKAYA N V, BOKATYI A N, GOLOVKIN A S, et al. Synthesis and characterization of novel succinyl chitosan-dexamethasone conjugates for potential intravitreal dexamethasone delivery[J]. Int J Mol Sci, 2021, 22(20): 10960.
|
| 18 |
YU A L, SHI H, LIU H, et al. Mucoadhesive dexamethasone-glycol chitosan nanoparticles for ophthalmic drug delivery[J]. Int J Pharm, 2020, 575: 118943.
|
| 19 |
YU X, ZHANG R, LEI L, et al. High drug payload nanoparticles formed from dexamethasone-peptide conjugates for the treatment of endotoxin-induced uveitis in rabbit[J]. Int J Nanomedicine, 2019, 14: 591-603.
|
| 20 |
IQBAL M, ZAFAR N, FESSI H, et al. Double emulsion solvent evaporation techniques used for drug encapsulation[J]. Int J Pharm, 2015, 496(2): 173-190.
|
| 21 |
NAVA-ARZALUZ M G, PIÑÓN-SEGUNDO E, GANEM-RONDERO A, et al. Single emulsion-solvent evaporation technique and modifications for the preparation of pharmaceutical polymeric nanoparticles[J]. Recent Pat Drug Deliv Formul,2012,6(3):209-223.
|
| 22 |
TAYLOR P N, ZHANG L, LEE R W J, et al. New insights into the pathogenesis and nonsurgical management of Graves orbitopathy[J]. Nat Rev Endocrinol, 2020, 16(2): 104-116.
|
| 23 |
LÄNGERICHT J, KRÄMER I, KAHALY G J. Glucocorticoids in Graves’ orbitopathy: mechanisms of action and clinical application[J]. Ther Adv Endocrinol Metab, 2020, 11. DOI:10.1177/2042018820958335 .
doi: 10.1177/2042018820958335
|
| 24 |
WANG Y J, DU B X, YANG M, et al. Peribulbar injection of glucocorticoids for thyroid-associated ophthalmopathy and factors affecting therapeutic effectiveness: a retrospective cohort study of 386 cases[J]. Exp Ther Med, 2020, 20(3): 2031-2038.
|
| 25 |
LI D P, SUN F Y. Observations on the efficacy of two methods for the treatment of upper eyelid retraction in thyroid-associated ophthalmopathy[J]. Biomed Res Int, 2021, 2021: 9514279.
|
| 26 |
HUEBERT I, HEINICKE N, KOOK D, et al. Dual platelet inhibition in cases of severe retrobulbar hemorrhage following retrobulbar and peribulbar anesthesia[J]. J Cataract Refract Surg, 2015, 41(10): 2092-2101.
|
| 27 |
ZHANG K R, TANG X, ZHANG J, et al. PEG-PLGA copolymers: their structure and structure-influenced drug delivery applications[J]. J Control Release, 2014, 183: 77-86.
|
| 28 |
NOORI KOOPAEI M, KHOSHAYAND M R, MOSTAFAVI S H, et al. Docetaxel loaded PEG-PLGA nanoparticles: optimized drug loading, in-vitro cytotoxicity and in-vivo antitumor effect[J]. Iran J Pharm Res, 2014, 13(3): 819-833.
|