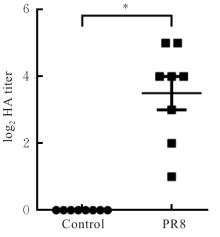
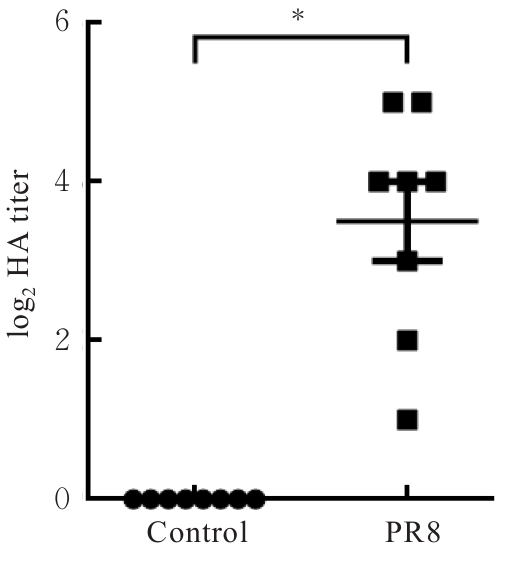
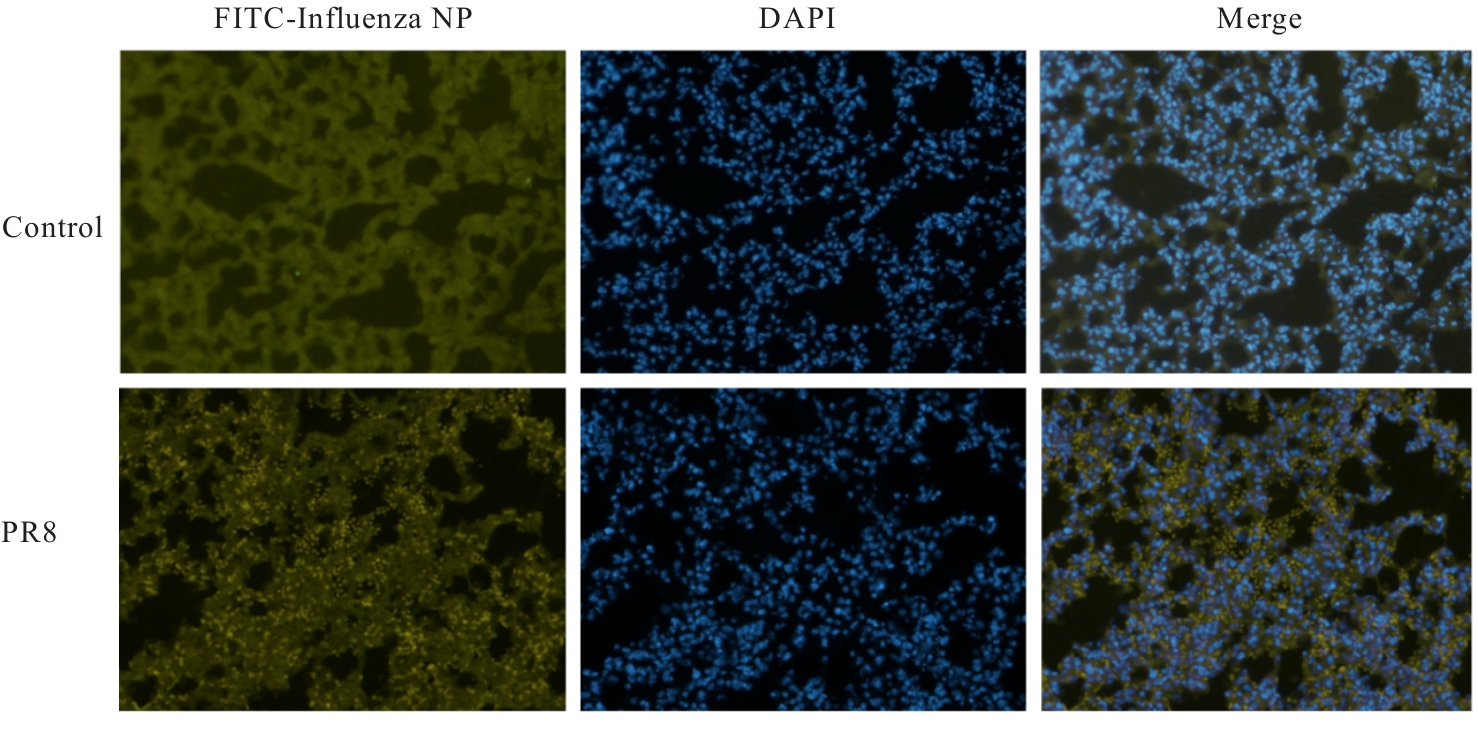
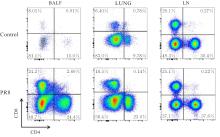
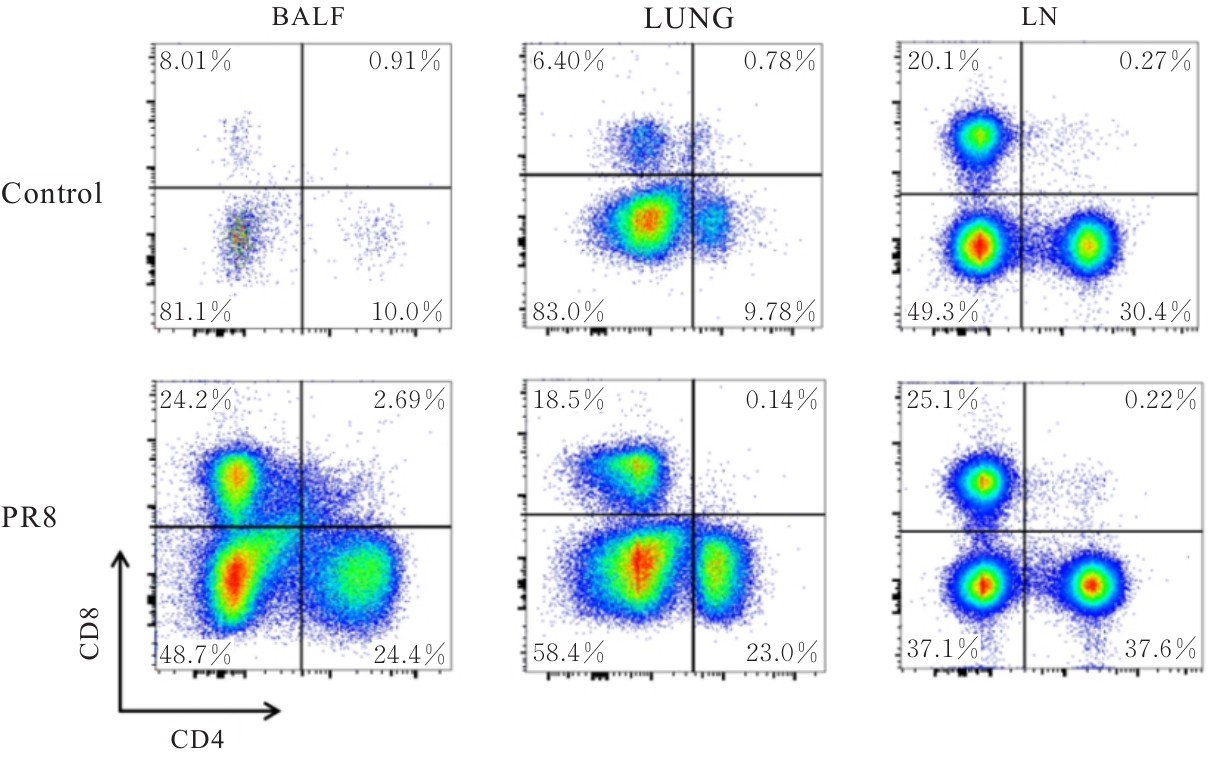
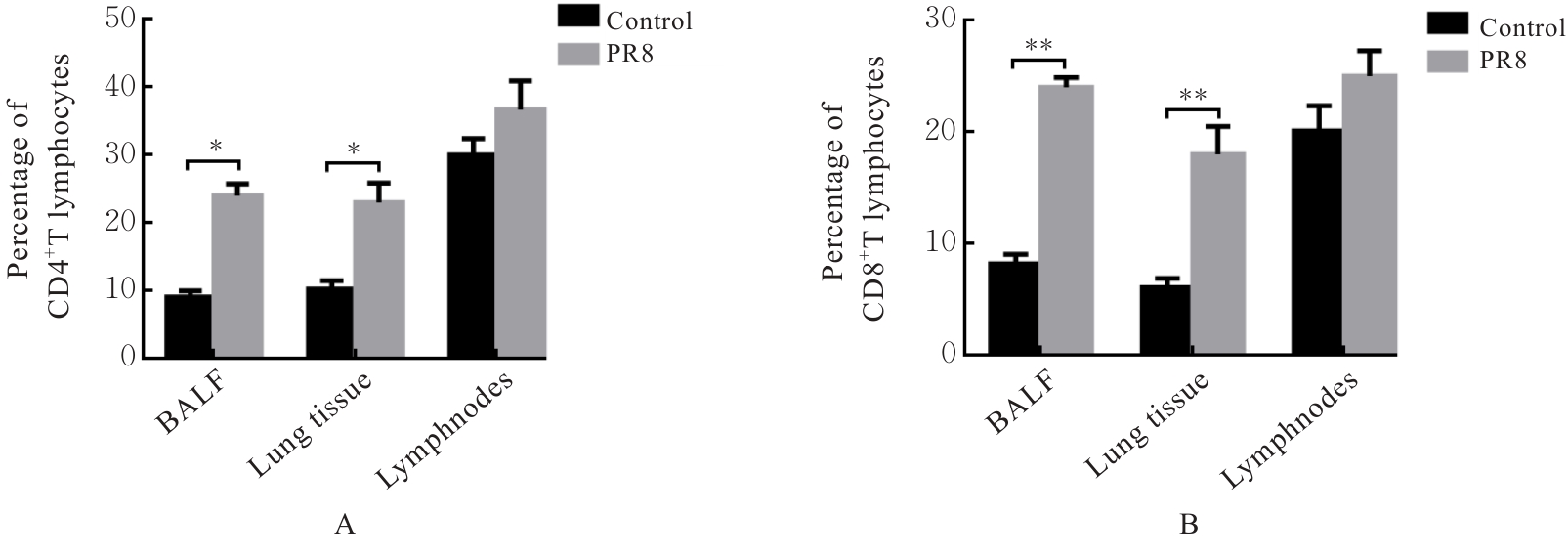
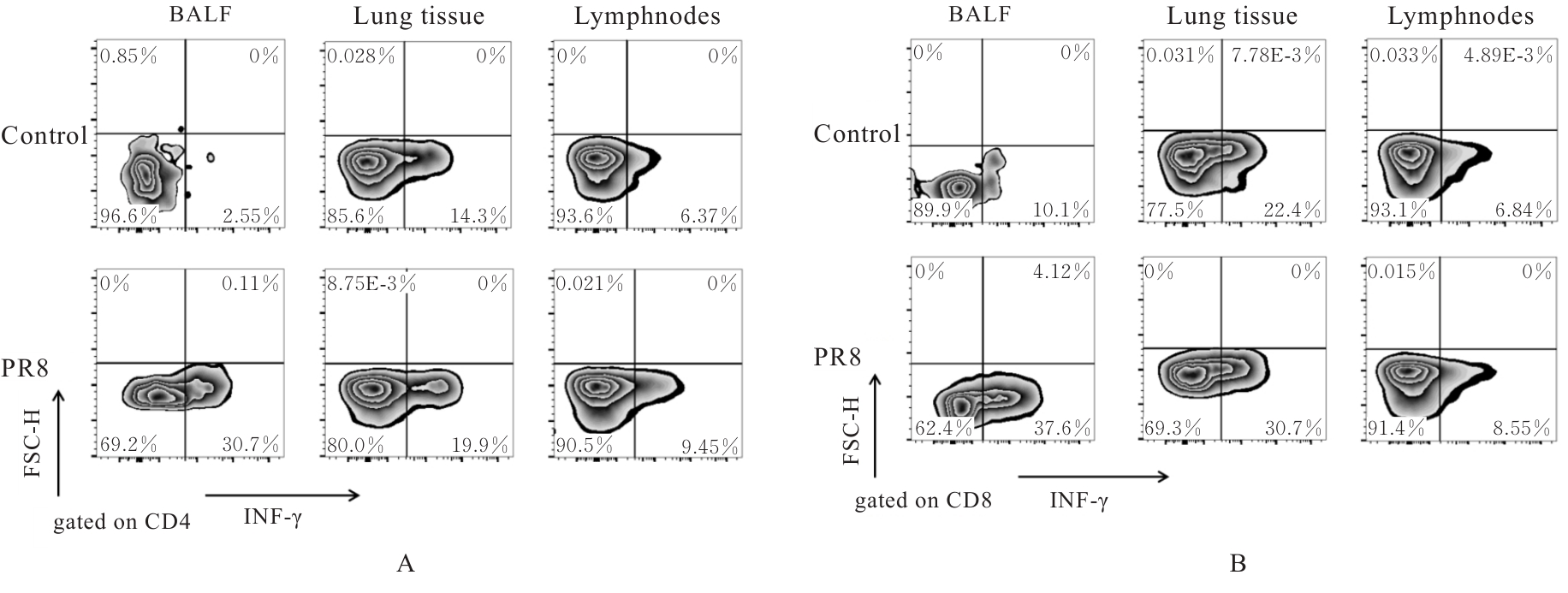
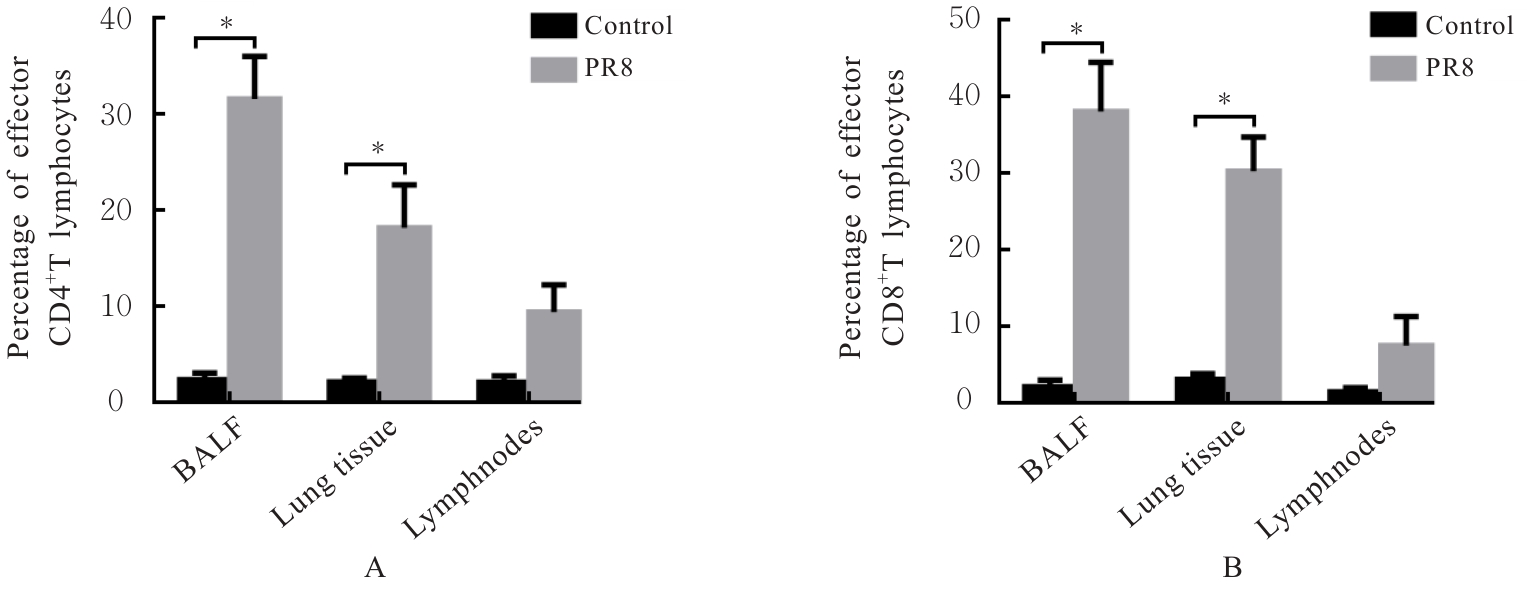
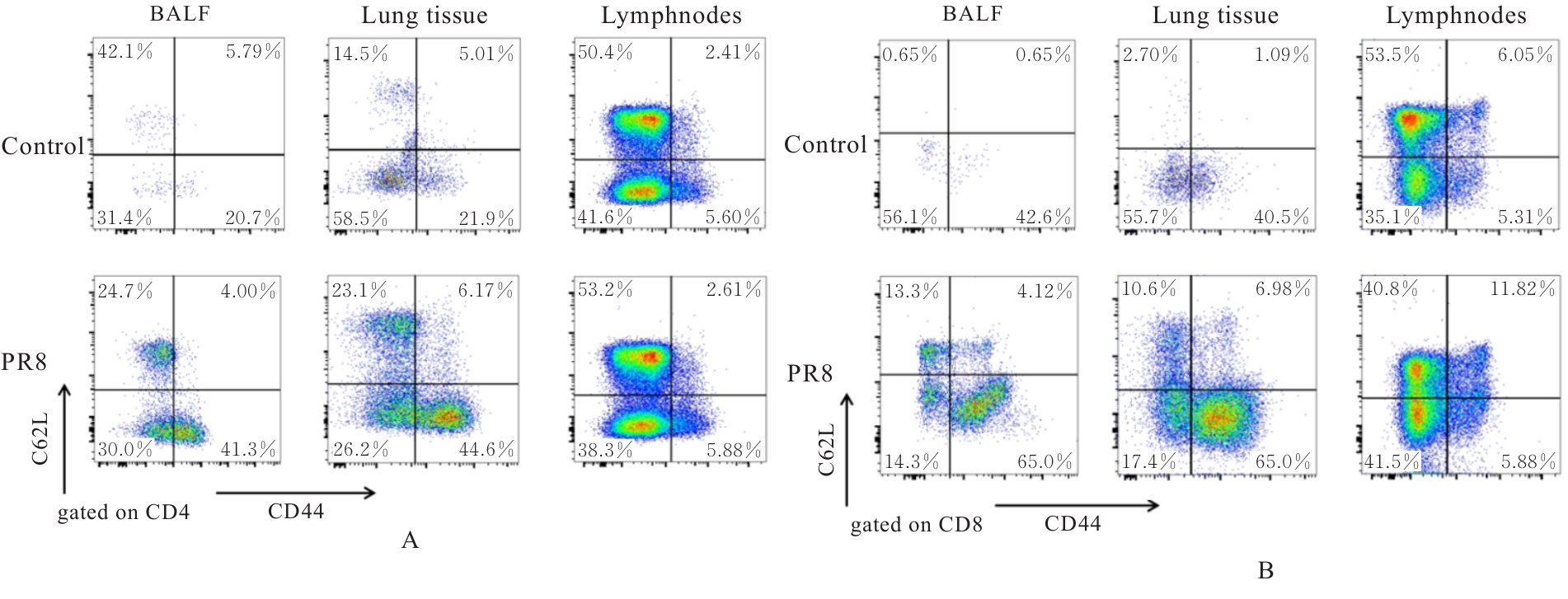
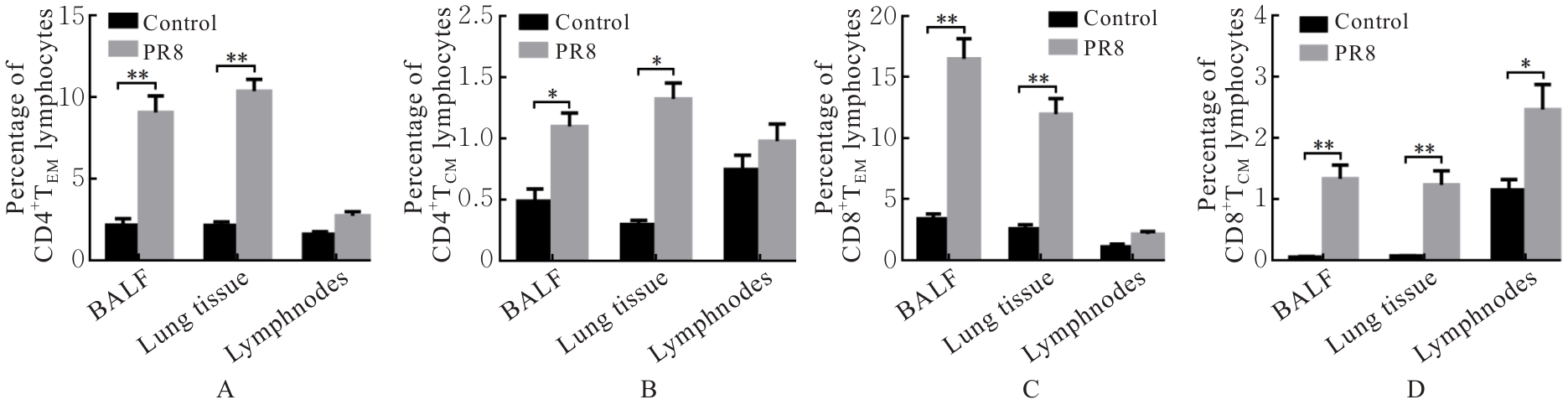
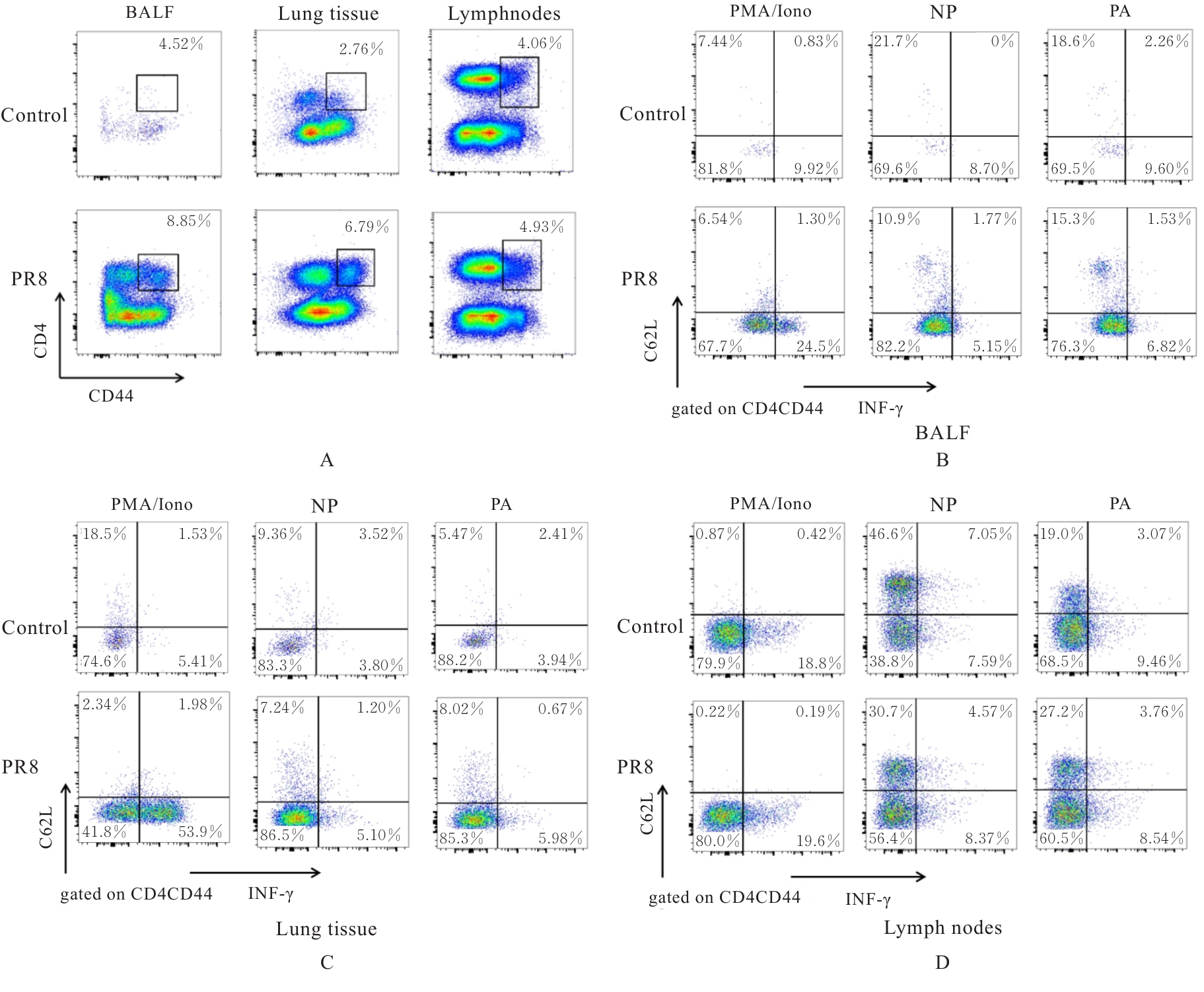
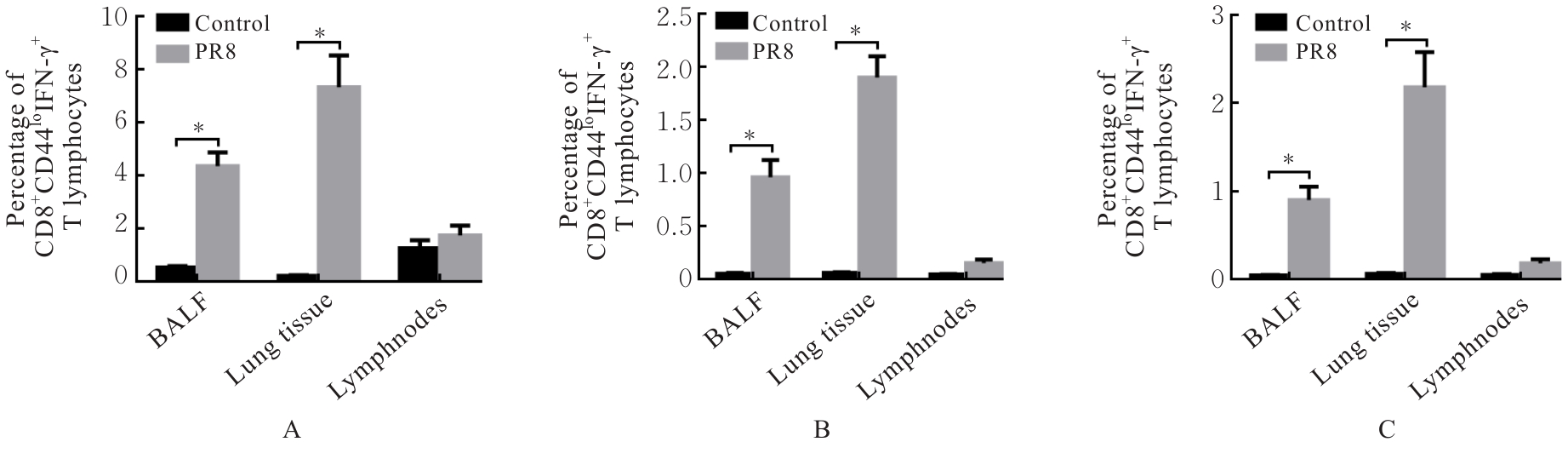
| [1] |
LI J L, ZHANG Y F, ZHANG X L, et al. Influenza and universal vaccine research in China[J]. Viruses, 2022, 15(1): 116.
|
| [2] |
LIANG Y Y. Pathogenicity and virulence of influenza[J]. Virulence, 2023, 14(1): 2223057.
|
| [3] |
KOSTOLANSKÝ F, TOMČÍKOVÁ K, BRIESTENSKÁ K, et al. Universal anti-influenza vaccines based on viral HA2 and M2e antigens[J]. Acta Virol, 2020, 64(4): 417-426.
|
| [4] |
SAYEDAHMED E E, ELSHAFIE N O, DOS SANTOS A P, et al. Development of NP-based universal vaccine for influenza a viruses[J]. Vaccines, 2024, 12(2): 157.
|
| [5] |
SUN X Y, MA H W, WANG X J, et al. Broadly neutralizing antibodies to combat influenza virus infection[J]. Antiviral Res, 2024, 221: 105785.
|
| [6] |
CEN L Q, ZHANG Z, SUN Y, et al. Efficacy of MAGE-A4 long peptide as a universal immunoprevention cancer vaccine[J]. Cancer Cell Int, 2024, 24(1): 232.
|
| [7] |
CORRADO M, PEARCE E L. Targeting memory T cell metabolism to improve immunity[J]. J Clin Invest, 2022, 132(1): e148546.
|
| [8] |
ZHOU J L, UDDBACK I, KOHLMEIER J E, et al. Vaccine induced memory CD8+ T cells efficiently prevent viral transmission from the respiratory tract[J]. Front Immunol, 2023, 14: 1322536.
|
| [9] |
ESSER M T, MARCHESE R D, KIERSTEAD L S, et al. Memory T cells and vaccines[J]. Vaccine, 2003, 21(5/6): 419-430.
|
| [10] |
DEEP D, GUDJONSON H, BROWN C C, et al. Precursor central memory versus effector cell fate and naïve CD4+ T cell heterogeneity[J]. J Exp Med, 2024, 221(10): e20231193.
|
| [11] |
GANNON P O, BAUMGAERTNER P, HUBER A, et al. Rapid and continued T-cell differentiation into long-term effector and memory stem cells in vaccinated melanoma patients[J]. Clin Cancer Res, 2017, 23(13): 3285-3296.
|
| [12] |
WELTEN S P M, ODERBOLZ J, YILMAZ V, et al. Influenza- and MCMV-induced memory CD8 T cells control respiratory vaccinia virus infection despite residence in distinct anatomical niches[J]. Mucosal Immunol, 2021, 14(3): 728-742.
|
| [13] |
MALOULI D, TIWARY M, GILBRIDE R M, et al. Cytomegalovirus vaccine vector-induced effector memory CD4+ T cells protect cynomolgus macaques from lethal aerosolized heterologous avian influenza challenge[J]. Nat Commun, 2024, 15(1): 6007.
|
| [14] |
FENG L, GAO Y Y, SUN M W, et al. The parallel presentation of two functional CTL epitopes derived from the O and Asia 1 serotypes of foot-and-mouth disease virus and swine SLA-2*HB01: implications for universal vaccine development[J]. Cells, 2022, 11(24): 4017.
|
| [15] |
LIU X J, ZHAO T Y, WANG L L, et al. Strategies targeting hemagglutinin cocktail as a potential universal influenza vaccine[J]. Front Microbiol, 2022, 13: 1014122.
|
| [16] |
MCGEE M C, HUANG W S. Evolutionary conservation and positive selection of influenza A nucleoprotein CTL epitopes for universal vaccination[J]. J Med Virol, 2022, 94(6): 2578-2587.
|
| [17] |
KIM S H, ESPAÑO E, PADASAS B T, et al. Influenza virus-derived CD8 T cell epitopes: implications for the development of universal influenza vaccines[J]. Immune Netw, 2024, 24(3): e19.
|
| [18] |
HASSAN M S H, SHARIF S. Immune responses to avian influenza viruses in chickens[J]. Virology, 2025, 603: 110405.
|
| [19] |
PARK S C, WIEST M J, YAN V, et al. Induction of protective immune responses at respiratory mucosal sites[J]. Hum Vaccin Immunother, 2024, 20(1): 2368288.
|
| [20] |
SCHREINER D, KING C G. CD4+ memory T cells at home in the tissue: mechanisms for health and disease[J]. Front Immunol, 2018, 9: 2394.
|
| [21] |
KEDZIERSKA K, VENTURI V, FIELD K, et al. Early establishment of diverse T cell receptor profiles for influenza-specific CD8+CD62L(hi) memory T cells[J]. Proc Natl Acad Sci U S A, 2006, 103(24): 9184-9189.
|