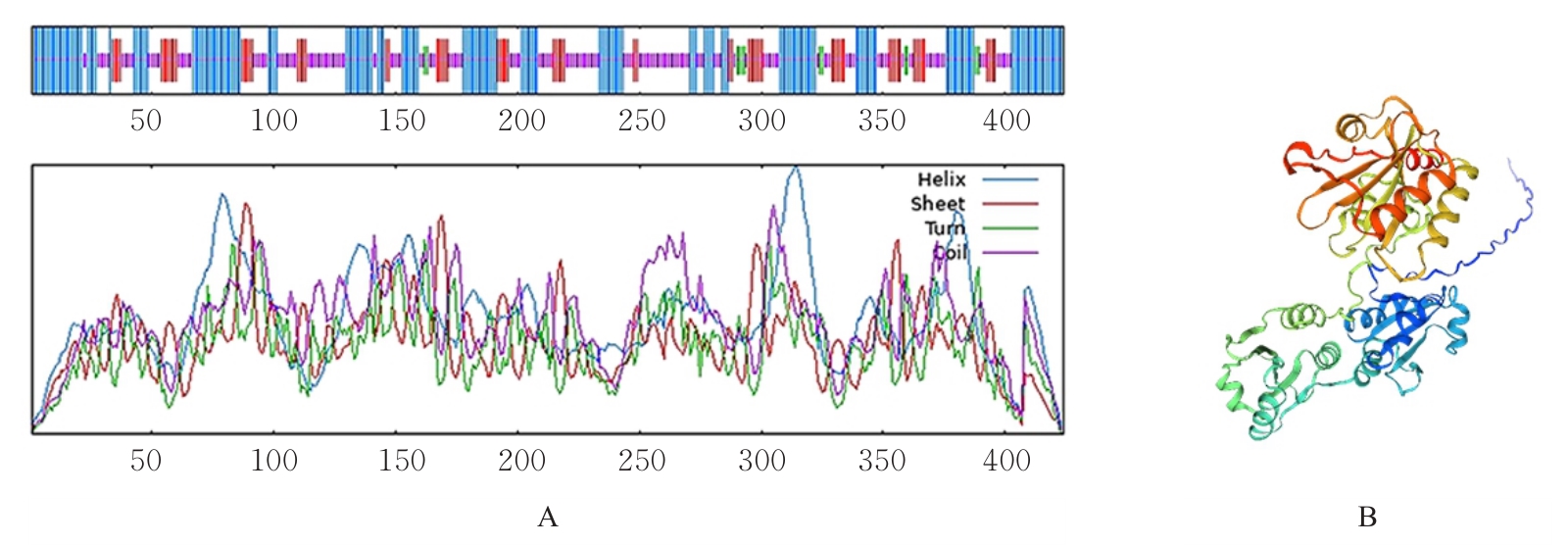
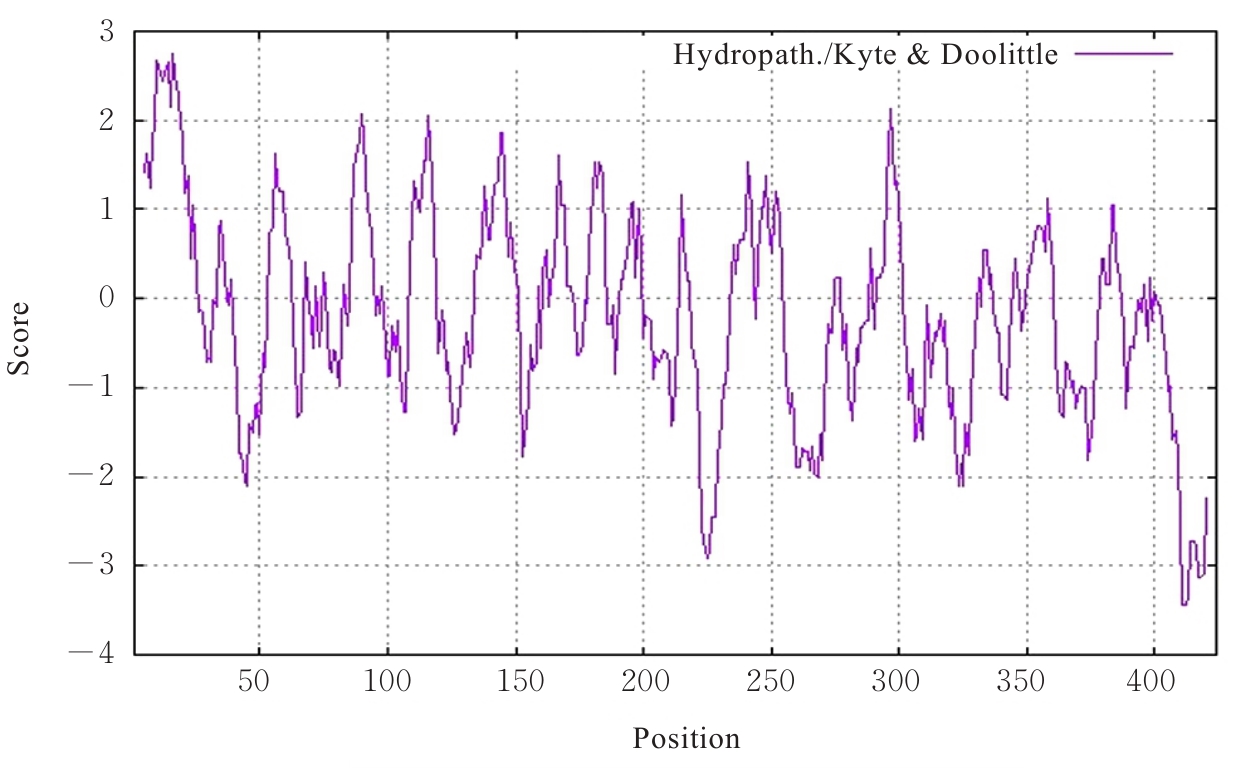
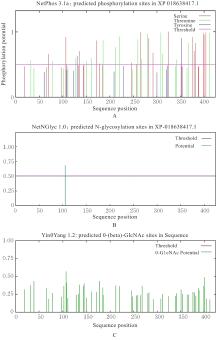
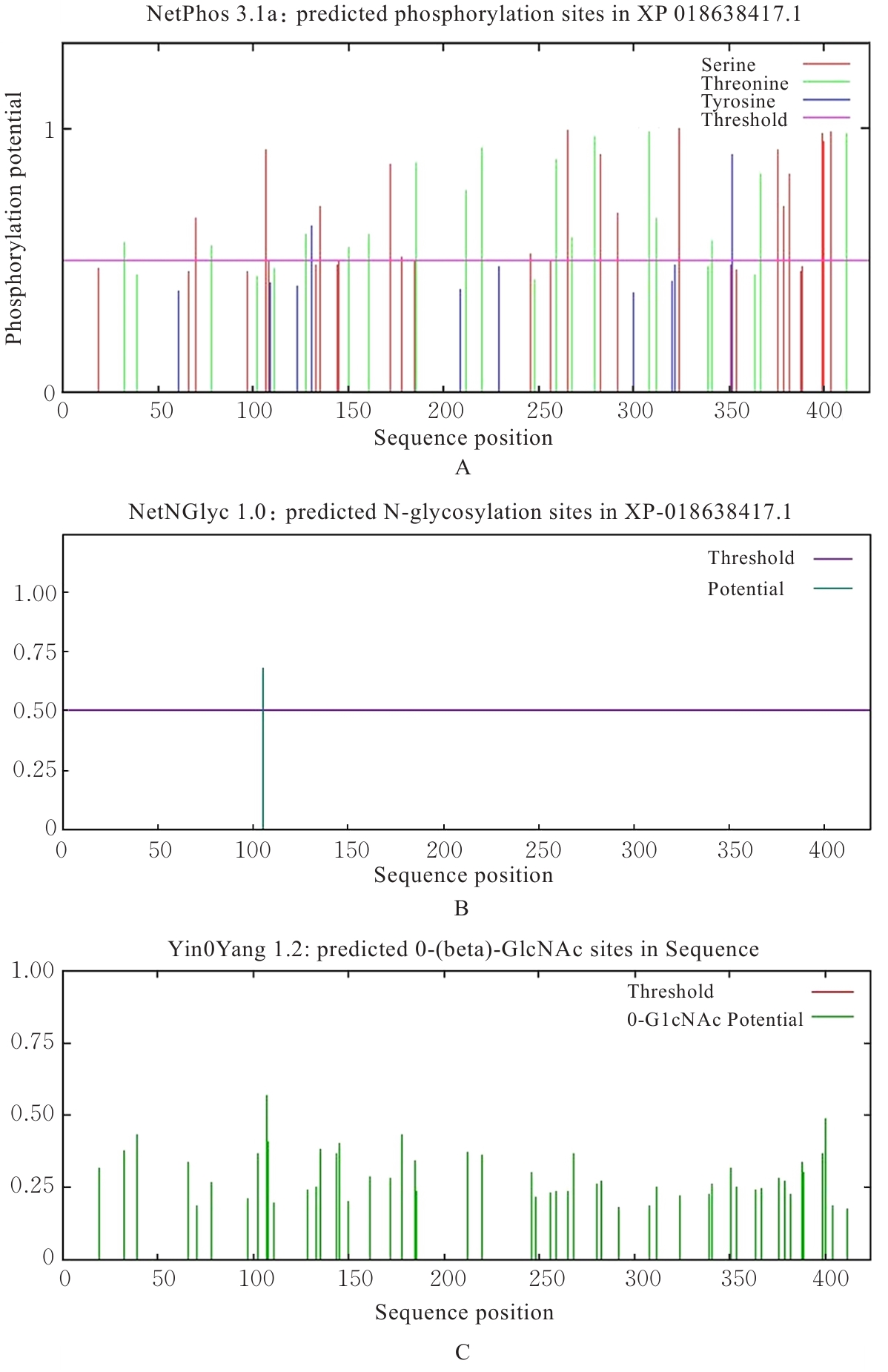
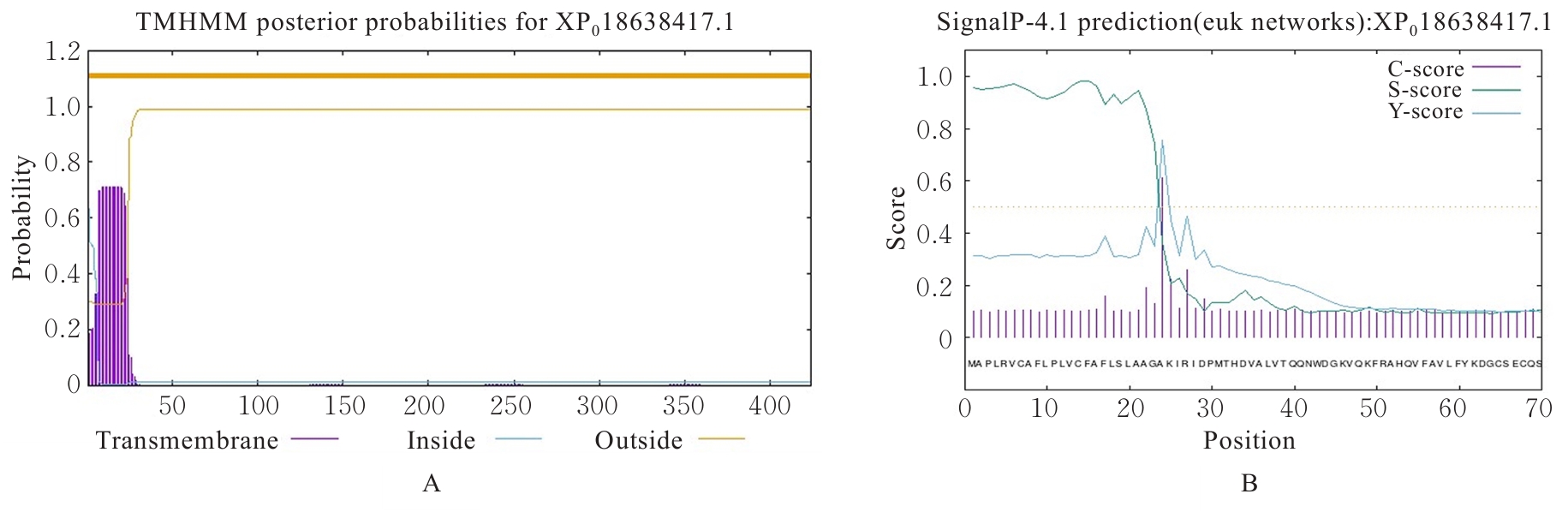
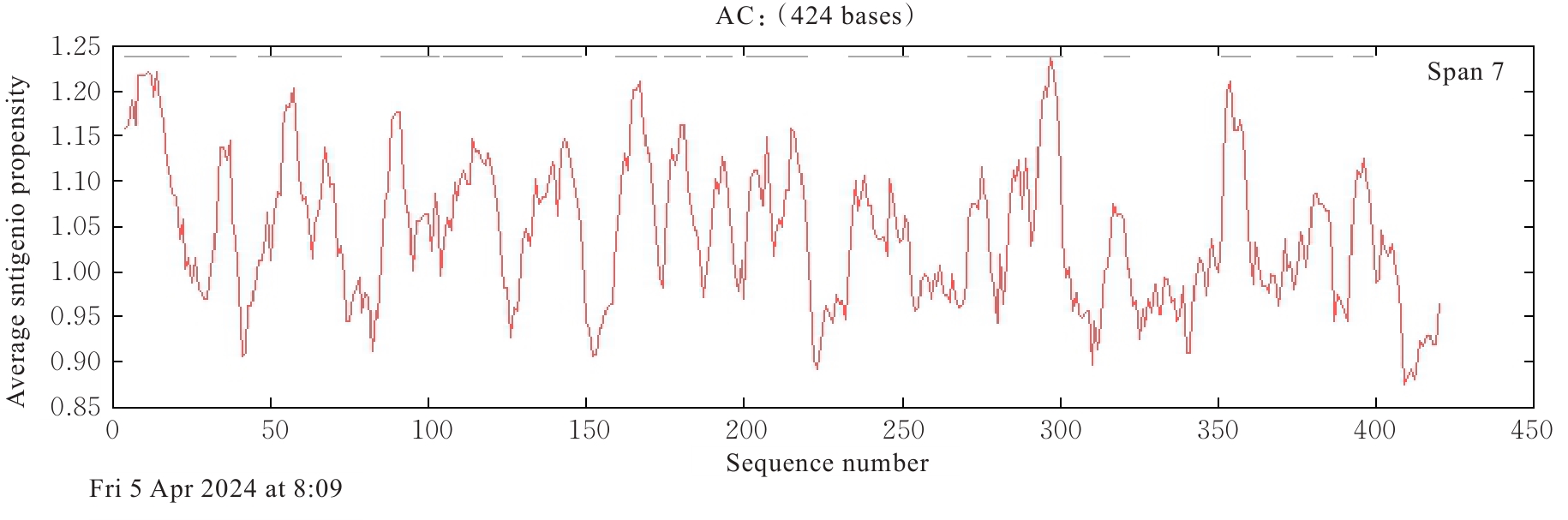
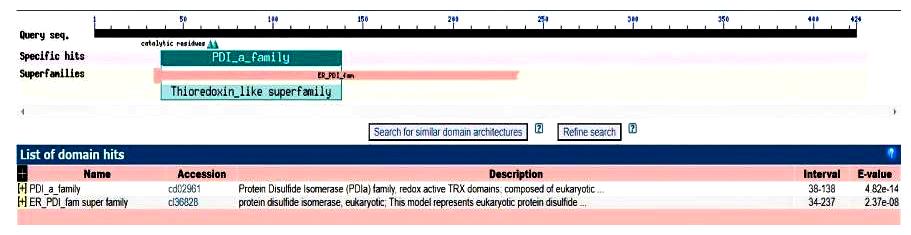
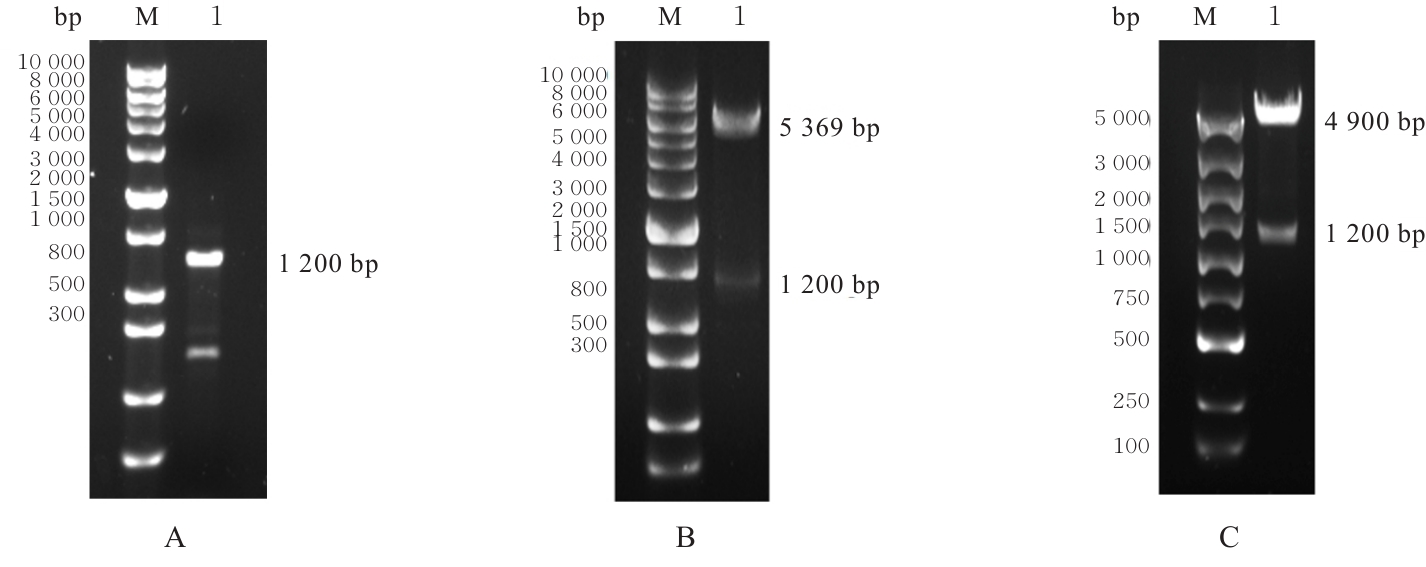
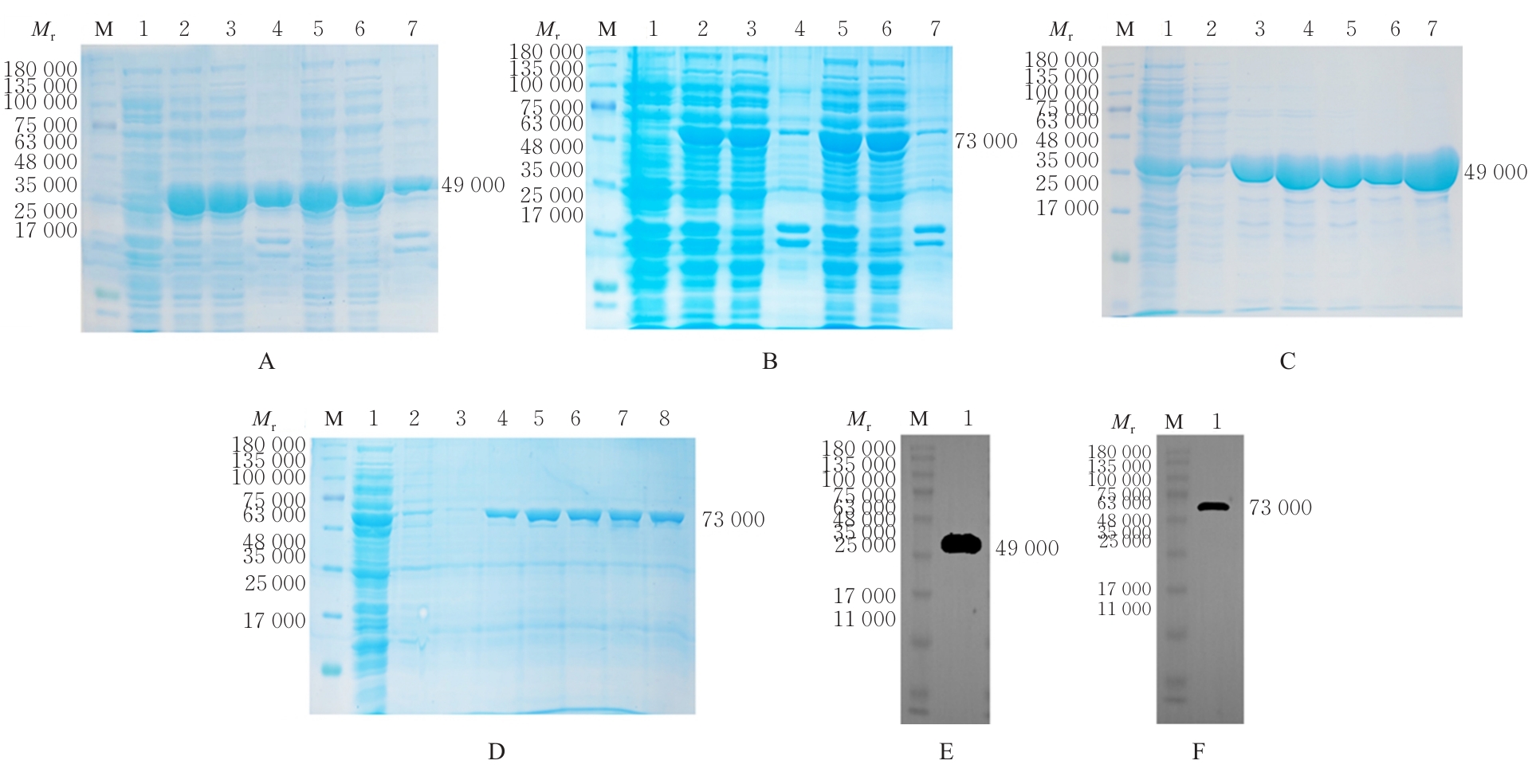
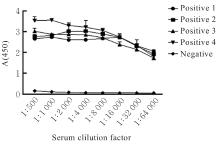
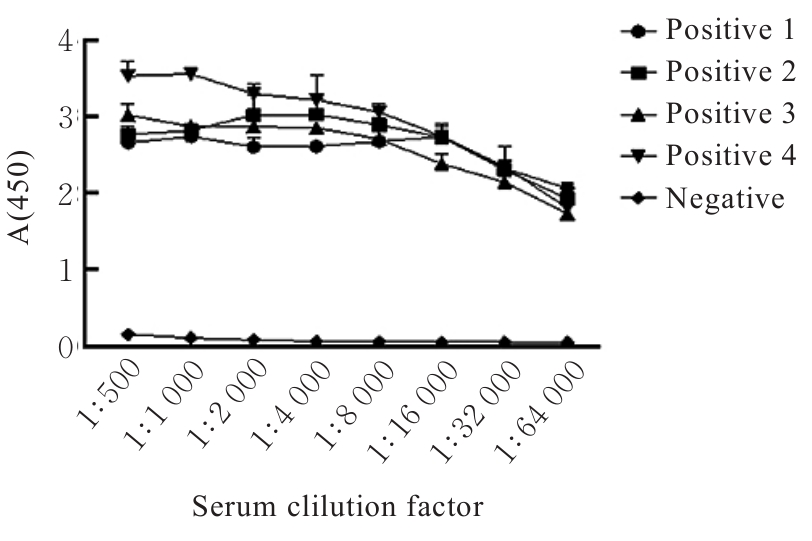
| [1] |
Wenning HE,Shaoqing FENG,Haiyao PANG,Jun MENG.
Dynamic expressions and subcellular localization of SGK3 mRNA and protein in oocytes of mice at different stages
[J]. Journal of Jilin University(Medicine Edition), 2023, 49(5): 1210-1216.
|
| [2] |
Jianyu ZHANG,Qionglin ZHANG.
Purification, crystallization and activity validation of oligoribonuclease from Pseudomonas aeruginosa
[J]. Journal of Jilin University(Medicine Edition), 2022, 48(5): 1124-1130.
|
| [3] |
LI Shimeng, ZHAO Lichun, QIAO Lu, HE Chengyan, LIU Zhuo.
Prokaryotic expressionof ATP5B and preparation and identification of its monoclonal antibody
[J]. Journal of Jilin University(Medicine Edition), 2019, 45(01): 184-189.
|
| [4] |
LIU Shaowei, JIANG Huan, WANG Xiaolong, LI Menghong, CHEN Yu, TANG Zhongyuan, HU Min.
Preparation and application of rabbit anti-osteoclastic protein tyrosine phosphatase polyclonal antibody
[J]. Journal of Jilin University Medicine Edition, 2018, 44(03): 661-666.
|
| [5] |
MENG Jun, HOU Yanjun, LIU Shan, FAN Shuzheng, HAN Yanqiu.
Effect of 14-3-3ε protein on localization of Cdc25B during meiotic resumption of mouse oocytes
[J]. Journal of Jilin University Medicine Edition, 2017, 43(06): 1080-1086.
|
| [6] |
LI Huimin, YI Huanfa.
Prokaryotic expression and purification of Toxoplasma gondii profilin protein
[J]. Journal of Jilin University Medicine Edition, 2017, 43(06): 1109-1114.
|
| [7] |
HUO Yunlong, WANG Chunyu, ZHAO Yue.
Subcellular localization of Mu2 protein and screening of its interacting proteins
[J]. Journal of Jilin University Medicine Edition, 2016, 42(06): 1054-1058.
|
| [8] |
YAN Yan, ZHANG Qian, MA Jing, LI Jianning, ZHANG Wei, SUN Yuning.
Preparation of polyclonal antibody against non-structural NP1 of minute virus of canine and its property identification
[J]. Journal of Jilin University Medicine Edition, 2015, 41(04): 864-869.
|
| [9] |
YANG Liu, WANG Xiaowan, WANG Bingyu, SONG Runmin, LI Jiang.
Recombination of GST-hZimp fusion protein and preparation of anti-hZimp10 antibody
[J]. Journal of Jilin University Medicine Edition, 2015, 41(03): 656-661.
|
| [10] |
LI Song,YANG Li-bin,ZHANG Shu-yan,JIN Yu,CHEN Xiu-hua,LI Xin,LIU Ling,LI Shu-lei,LI Shu-hong.
Prokaryotic expression and identification of recombinant Cystatin of Cyprinus carpio var.Jian and preparation of its polyclonal antibody
[J]. Journal of Jilin University Medicine Edition, 2014, 40(01): 204-209.
|
| [11] |
HE Qiu-li,ZHANG Xu,WANG Juan,ZHAI Rui-ping,SUN Xia-xia,QIAO Cai-xia, NI Wei-hua,GAO Su-jun,TAI Gui-xiang.
Establishment of indirect sandwich ELISA methodfor serum MUC1 detection and its application
in diagnosis of lung cancer
[J]. Journal of Jilin University Medicine Edition, 2013, 39(2): 400-404.
|
| [12] |
ZHANG Lei,ZHANG Shi-tao,ZENG Xian-yang,ZHANG Xiao-ping,HAO Fan-fan,WANG Feng,LI Wan-nan,FU Xue-qi.
Cloning and expression of megakaryocyte protein tyrosine phosphatase 2 and its relationship with osteoporosis
[J]. Journal of Jilin University Medicine Edition, 2013, 39(2): 227-231.
|
| [13] |
FANG Zhen,JU Wen,QIN Ya-nan,MENG Ri-zeng,PAN Feng-guang.
Preparation and evaluation of FITC labeled Escherichia coli O157∶H7polyclonal antibody
[J]. Journal of Jilin University Medicine Edition, 2013, 39(1): 165-169.
|
| [14] |
JU Wen,SONG Xiu-ling,YUAN Ye,ZHANG Shi-yao,FANG Zhen,XU Kun,LI Juan.
Preparation of anti-Listeria monocytogenes polyclonal antibody and its application in latex agglutination test
[J]. J4, 2012, 38(5): 821-826.
|
| [15] |
YANG Na-Yang, JIU Xuan, WU Xian-Jun, LIU Jin-Mei, SHI Yi-Lang, LI Xiao-Meng.
Construction of small ubiquitin-like modifiers SUMO3/pGEX-4T-1 plasmid and expression of GST/SUMO3 fusion protein in E.coli
[J]. J4, 2011, 37(6): 1010-1014.
|
 )
)