| 1 |
SETTEMBRE C, DE CEGLI R, MANSUETO G,et al.TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop[J]. Nat Cell Biol, 2013, 15(6): 647-658.
|
| 2 |
RAI S N, TIWARI N, SINGH P, et al. Therapeutic potential of vital transcription factors in Alzheimer’s and Parkinson’s disease with particular emphasis on transcription factor EB mediated autophagy[J]. Front Neurosci, 2021, 15: 777347.
|
| 3 |
DECRESSAC M, MATTSSON B, WEIKOP P,et al. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity[J]. Proc Natl Acad Sci U S A, 2013, 110(19): E1817-E1826.
|
| 4 |
WYVEKENS N, RECHSTEINER M, FRITZ C,et al. Histological and molecular characterization of TFEB-rearranged renal cell carcinomas[J]. Virchows Arch, 2019, 474(5): 625-631.
|
| 5 |
HARADA S, CALIÒ A N, JANOWSKI K M, et al. Diagnostic utility of one-stop fusion gene panel to detect TFE3/TFEB gene rearrangement and amplification in renal cell carcinomas[J]. Mod Pathol, 2021, 34(11): 2055-2063.
|
| 6 |
PUERTOLLANO R, FERGUSON S M, BRUGAROLAS J, et al. The complex relationship between TFEB transcription factor phosphorylation and subcellular localization[J]. EMBO J, 2018, 37(11): e98804.
|
| 7 |
PAQUETTE M, EL-HOUJEIRI L, ZIRDEN L C,et al.AMPK-dependent phosphorylation is required for transcriptional activation of TFEB and TFE3[J]. Autophagy, 2021, 17(12): 3957-3975.
|
| 8 |
PALMIERI M, PAL R, NELVAGAL H R, et al. mTORC1-independent TFEB activation via Akt inhibition promotes cellular clearance in neurodegenerative storage diseases[J]. Nat Commun, 2017, 8: 14338.
|
| 9 |
HSU C L, LEE E X, GORDON K L, et al. MAP4K3 mediates amino acid-dependent regulation of autophagy via phosphorylation of TFEB[J]. Nat Commun, 2018, 9(1): 942.
|
| 10 |
YIN Q Y, JIAN Y L, XU M, et al. CDK4/6 regulate lysosome biogenesis through TFEB/TFE3[J]. J Cell Biol, 2020, 219(8): e201911036.
|
| 11 |
SAKELLARIOU D, TIBERTI M, KLEIBER T H,et al.eIF4A3 regulates the TFEB-mediated transcriptional response via GSK3B to control autophagy[J]. Cell Death Differ, 2021, 28(12): 3344-3356.
|
| 12 |
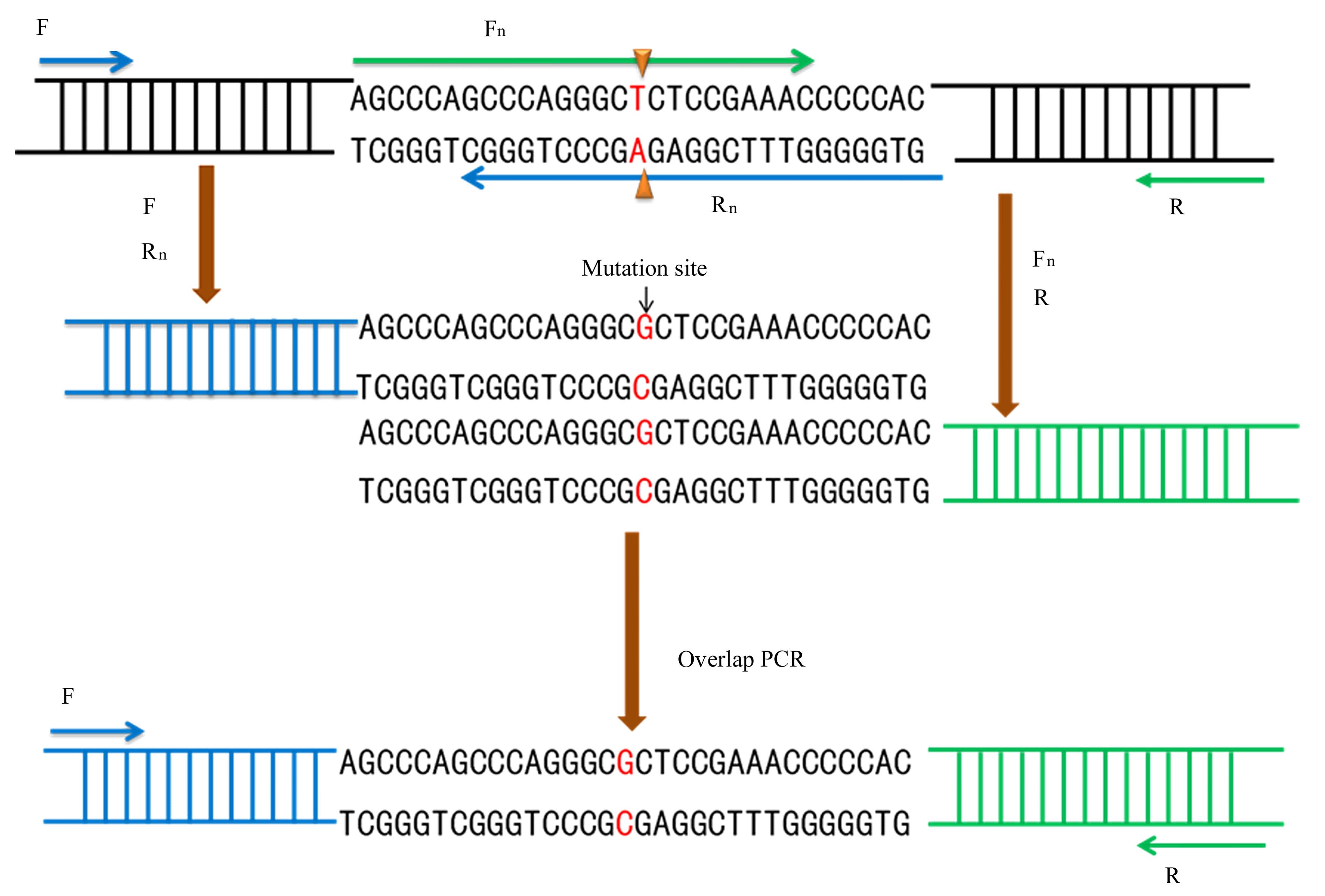
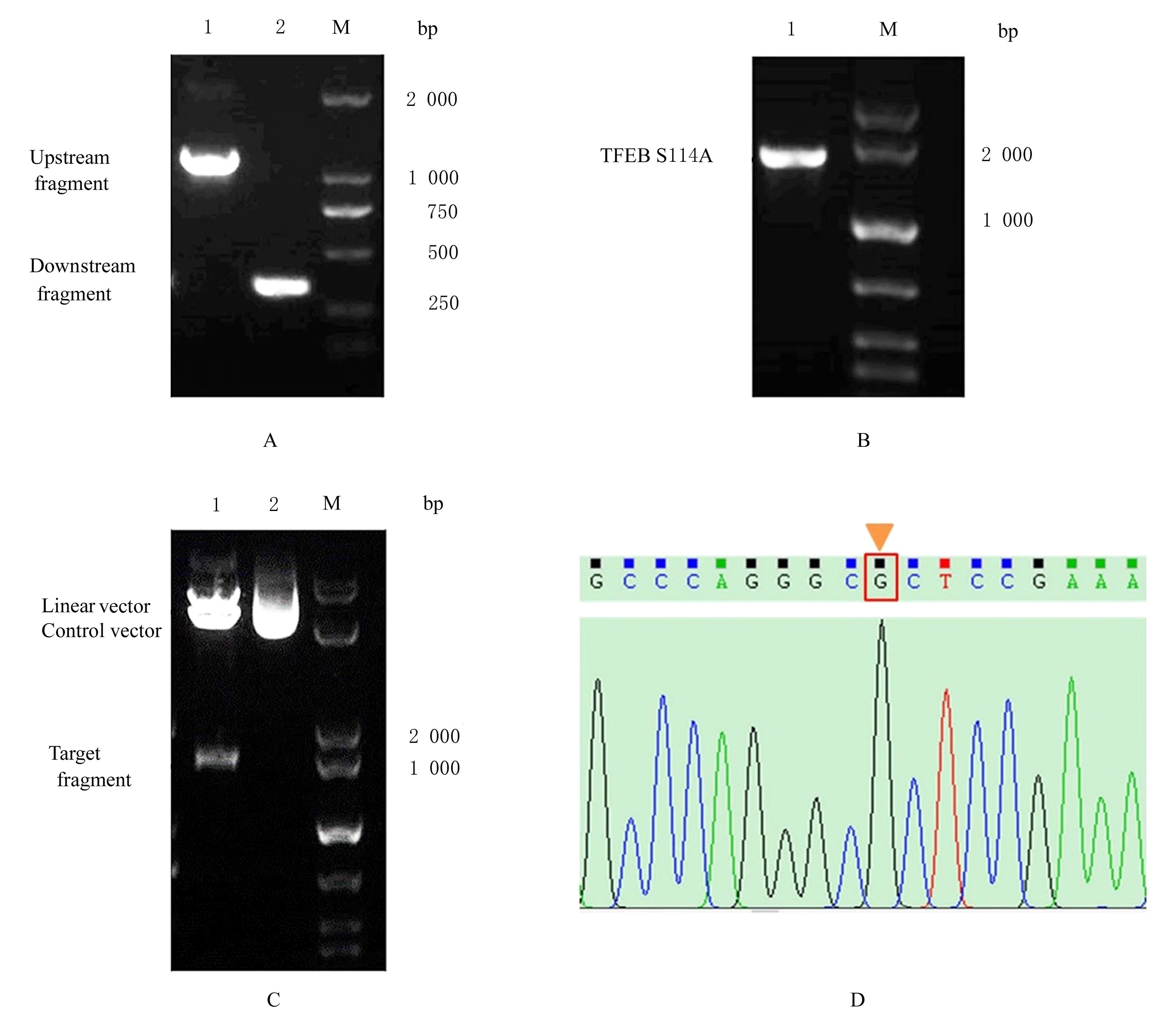
BRYKSIN A, MATSUMURA I. Overlap extension PCR cloning[J]. Methods Mol Biol, 2013, 1073: 31-42.
|
| 13 |
XIAO J, MA M Q, LIANG M X, et al. Site-directed mutagenesis of long gene by partial amplification combining with double fragments ligation[J]. Sheng Wu Gong Cheng Xue Bao, 2020, 36(6): 1232-1240.
|
| 14 |
MEDINA D L, DI PAOLA S, PELUSO I, et al. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB[J]. Nat Cell Biol, 2015, 17(3): 288-299.
|
| 15 |
LIU Y Y, XUE X, ZHANG H T, et al. Neuronal-targeted TFEB rescues dysfunction of the autophagy-lysosomal pathway and alleviates ischemic injury in permanent cerebral ischemia[J].Autophagy,2019,15(3): 493-509.
|
| 16 |
FANG Y Y, JI L L, ZHU C Y, et al. Liraglutide alleviates hepatic steatosis by activating the TFEB-regulated autophagy-lysosomal pathway[J]. Front Cell Dev Biol, 2020, 8: 602574.
|
| 17 |
SARDIELLO M. Transcription factor EB: from master coordinator of lysosomal pathways to candidate therapeutic target in degenerative storage diseases[J]. Ann N Y Acad Sci, 2016, 1371(1): 3-14.
|
| 18 |
ROCZNIAK-FERGUSON A, PETIT C S, FROEHLICH F, et al. The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis[J]. Sci Signal, 2012, 5(228): ra42.
|
| 19 |
DECRESSAC M, BJÖRKLUND A. TFEB: Pathogenic role and therapeutic target in Parkinson disease[J]. Autophagy, 2013, 9(8): 1244-1246.
|
| 20 |
SHA Y B, RAO L, SETTEMBRE C, et al. STUB1 regulates TFEB-induced autophagy-lysosome pathway[J]. EMBO J, 2017, 36(17): 2544-2552.
|
| 21 |
SETTEMBRE C, DI MALTA C, POLITO V A,et al. TFEB links autophagy to lysosomal biogenesis[J]. Science, 2011, 332(6036): 1429-1433.
|
| 22 |
戴 灿, 苗聪秀, 卢光琇. 基于重叠延伸PCR法的定点突变技术[J].现代生物医学进展,2010,10(3): 411-412.
|
| 23 |
WARRENS A N, JONES M D, LECHLER R I. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest[J]. Gene, 1997, 186(1): 29-35.
|
| 24 |
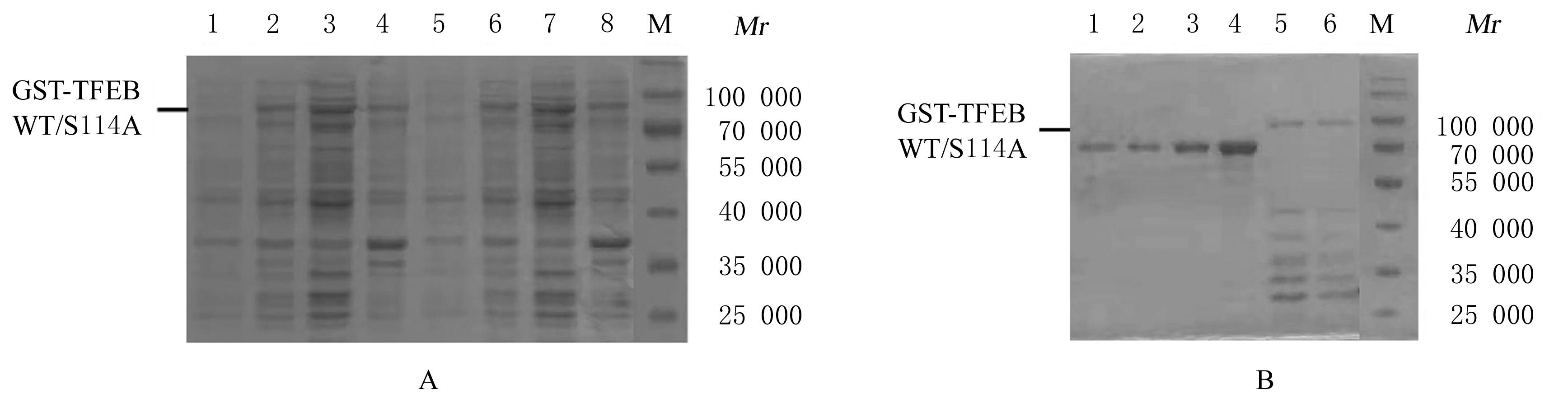
叶姣, 陈长华, 夏杰, 等. 温度对重组大肠杆菌生长及外源蛋白表达的影响[J]. 华东理工大学学报, 2002, 28(4): 364-367.
|
| 25 |
任增亮, 堵国成, 陈坚, 等. 大肠杆菌高效表达重组蛋白策略[J].中国生物工程杂志,2007,27(9): 103-109.
|
| 26 |
TURNER P, HOLST O, KARLSSON E N. Optimized expression of soluble cyclomaltodextrinase of thermophilic origin in Escherichia coli by using a soluble fusion-tag and by tuning of inducer concentration[J]. Protein Expr Purif, 2005, 39(1):54-60.
|
 ),Junjie LIU,Siwei LU,Dongjun JIANG
),Junjie LIU,Siwei LU,Dongjun JIANG