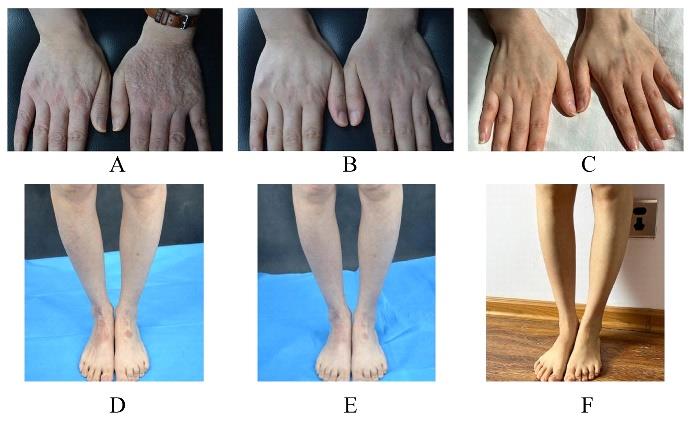
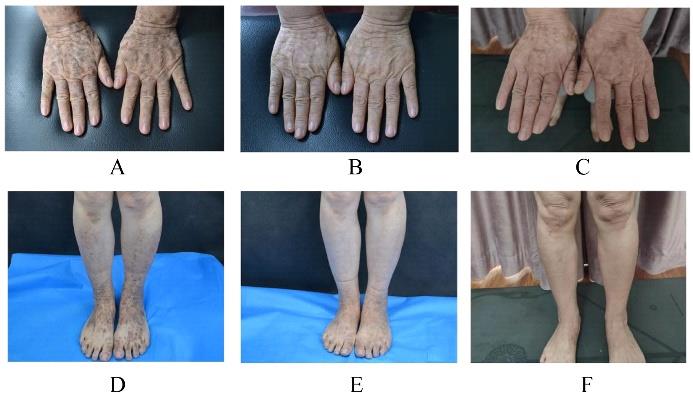
| 1 |
中华医学会皮肤性病学分会免疫学组, 特应性皮炎协作研究中心. 中国特应性皮炎诊疗指南(2020版)[J]. 中华皮肤科杂志, 2020, 53(2): 81-88.
|
| 2 |
JOHNSON B B, FRANCO A I, BECK L A, et al. Treatment-resistant atopic dermatitis: challenges and solutions[J]. Clin Cosmet Investig Dermatol, 2019, 12: 181-192.
|
| 3 |
中华医学会皮肤性病学分会特应性皮炎研究中心, 中华医学会皮肤性病学分会儿童学组. 度普利尤单抗治疗特应性皮炎专家共识[J].中华皮肤科杂志, 2022, 55(6): 465-470.
|
| 4 |
刘 擘, 宋晓婷, 李若瑜, 等. 度普利尤单抗治疗特应性皮炎的疗效及安全性分析[J]. 中华皮肤科杂志, 2022, 55(4): 295-298.
|
| 5 |
顾建青, 高 翔, 孙劲旅, 等. 度普利尤单抗治疗中重度特应性皮炎初步临床观察[J]. 中华临床免疫和变态反应杂志, 2021, 15(4): 370-375.
|
| 6 |
黄馨,陈筱昀,李亚萍,等. 度普利尤单抗治疗123例特应性皮炎的疗效及安全性分析[J]. 中华皮肤科杂志,2022,55(6):486-493.
|
| 7 |
LIU P, ZHAO Y, MU ZL, et al. Clinical features of adult/adolescent atopic dermatitis and Chinese criteria for atopic dermatitis[J].Chin Med J(Engl),2016,129(7):757-762.
|
| 8 |
LEE D D, HUANG C K, KO P C, et al. Association of primary cutaneous amyloidosis with atopic dermatitis: a nationwide population-based study inTaiwan [J]. Br J Dermatol, 2011, 164(1): 148-153.
|
| 9 |
CHIA B, TAN A, TEY H L. Primary localized cutaneous amyloidosis: association with atopic dermatitis[J].J Eur Acad Dermatol Venereol,2014,28(6): 810-813.
|
| 10 |
SIMON H U, ROTHENBERG M E, BOCHNER B S, et al. Refining the definition of hypereosinophilic syndrome[J]. J Allergy Clin Immunol, 2010, 126(1): 45-49.
|
| 11 |
赵作涛, 高兴华. 中重度特应性皮炎系统药物达标治疗专家指导建议[J]. 中国皮肤性病学杂志,2022, 36(8): 855-864.
|
| 12 |
GUTTMAN-YASSKY E, BISSONNETTE R, UNGAR B, et al. Dupilumab progressively improves systemic and cutaneous abnormalities in patients with atopic dermatitis[J].J Allergy Clin Immunol, 2019, 143(1): 155-172.
|
| 13 |
AOKI K, OHYAMA M, MIZUKAWA Y. A case of lichen amyloidosis associated with atopic dermatitis successfully treated with dupilumab: a case report and literature review[J]. Dermatol Ther, 2021, 34(4): e15005.
|
| 14 |
SCHREML S. Is targeting interleukin-31 a cure for the itch? Lessons from amyloidosis[J]. Br J Dermatol, 2016, 174(6): 1192-1193.
|
| 15 |
GARCOVICH S, MAURELLI M, GISONDI P, et al. Pruritus as a distinctive feature of type 2 inflammation[J]. Vaccines, 2021, 9(3): 303.
|
| 16 |
BANGERT C, RINDLER K, KRAUSGRUBER T, et al. Persistence of mature dendritic cells, TH2A, and Tc2 cells characterize clinically resolved atopic dermatitis under IL-4Rα blockade[J]. Sci Immunol, 2021, 6(55): eabe2749.
|
| 17 |
WORM M, SIMPSON E L, THAÇI D, et al. Efficacy and safety of multiple dupilumab dose regimens after initial successful treatment in patients with atopic dermatitis: a randomized clinical trial[J]. JAMA Dermatol, 2020, 156(2): 131-143.
|
| 18 |
MIZUNO M, HORIGUCHI G, TERAMUKAI S,et al. Association study of transition of laboratory marker levels and transition of disease activity of atopic dermatitis patients treated with dupilumab[J]. Australas J Dermatol, 2021, 62(4): e504-e509.
|
| 19 |
NETTIS E, MASCIOPINTO L, LEO E D, et al. Dupilumab elicits a favorable response in type-2 inflammatory comorbidities of severe atopic dermatitis[J]. Clin Mol Allergy, 2021, 19(1): 9.
|
| 20 |
张佩莲,叶建州.度普利尤单抗治疗特应性皮炎的不良反应与防治对策[J/OL].中国皮肤性病学杂志,2023.DOI:10.13735/j.cjdv.1001-7089.202207104 .
doi: 10.13735/j.cjdv.1001-7089.202207104
|
| 21 |
BETTUZZI T, DRUCKER A, STAUMONT-SALLÉ D, et al. Adverse events associated with dupilumab in the World Health Organization pharmacovigilance database[J]. J Am Acad Dermatol, 2022, 86(2): 431-433.
|
| 22 |
DELEURAN M, THAÇI D, BECK L A, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study[J]. J Am Acad Dermatol, 2020, 82(2): 377-388.
|
 )
)