吉林大学学报(工学版) ›› 2022, Vol. 52 ›› Issue (1): 1-24.doi: 10.13229/j.cnki.jdxbgxb20210047
• 综述 •
钒氧化还原流电池技术综述
- 1.吉林大学 汽车工程学院,长春 130022
2.中鼎(吉林)智能制造工程有限公司,长春 130062
Review of vanadium redox flow battery technology
Da-wei QU1( ),Fan YANG1,Lu-yan FAN1,Xiao-yu FENG2,Jia-yi MA1(
),Fan YANG1,Lu-yan FAN1,Xiao-yu FENG2,Jia-yi MA1( )
)
- 1.College of Automotive Engineering,Jilin University,Changchun 130022,China
2.Zhong Ding Intelligent Manufacturing Engineering Co. ,Ltd. ,Changchun 130062,China
摘要:
钒氧化还原流电池(VRFB)具有响应速度快、储能量巨大、成本低、效率高、使用寿命长和低污染等特点,在大型储能系统领域有着广泛的应用潜力。虽然钒氧化还原流电池已被商用,但是其能量密度与效率仍然受电极活性、温度稳定性、膜内交叉污染和极化损失等因素的限制。针对当前钒氧化还原流电池领域的研究热点,从电池的电极、电解液、隔膜以及双极板流场4个方面分别展开综述,阐述VRFB技术的工作原理,介绍VRFB不同组件及其最新的研究现状,总结了限制VRFB进一步发展的技术因素,探讨这些限制的解决方法,并对VRFB技术今后的发展进行了展望与分析。
中图分类号:
- TK02
| 1 | Bhattacharjee A, Saha H. Development of an efficient thermal management system for vanadium redox flow battery under different charge-discharge conditions[J]. Appl Energy, 2018, 230: 1182-1192. |
| 2 | Duan Z N, Qu Z G, Wang Q, et al. Structural modification of vanadium redox flow battery with high electrochemical corrosion resistance[J]. Appl Energy, 2019, 250: 1632-1640. |
| 3 | 林立乾, 米增强, 贾雨龙, 等. 面向电力市场的分布式储能聚合参与电网调峰[J]. 储能科学与技术, 2019, 8(2): 276-283. |
| Lin Li-qian, Mi Zeng-qiang, Jia Yu-long, et al. Distributed energy storage aggregation for power grid peak shaving in a power market[J]. Energy Storage Science and Technology, 2019, 8(2): 276-283. | |
| 4 | Diaz-Gonzalez F, Sumper A, Gomis-Bellmunt O, et al. A review of energy storage technologies for wind power applications[J]. Renewable and Sustainable Energy Reviews, 2012, 16(4): 2154-2171. |
| 5 | Skyllas-Kazacos M, Chakrabarti M H, Hajimolana S A, et al. Progress in flow battery research and development[J]. Journal of the Electrochemical Society, 2011, 158(8): 55-79. |
| 6 | 苏俊臣. 全钒液流电池用氮磷共掺杂石墨毡电极的研究[D]. 北京:清华大学化学学院, 2018. |
| Su Jun-chen. Research on nitrogen and phosphorus co-doped graphite felt electrodes for vanadium flow battery[D]. Beijing: College of Chemistry, Tsinghua University, 2018. | |
| 7 | 吴雨森. 全钒液流电池SOC及能量管理系统研究[D]. 合肥:合肥工业大学电气与自动化工程学院, 2019. |
| Wu Yu-sen. Research on SOC estimation and energy management system of all vanadium redox flow battery[D]. Heifei:School of Electrical Engineering and Automation, Heifei University of Technology, 2019. | |
| 8 | Aramendia I, Fernandez-Gamiz U, Martinez-San-Vicente A, et al. Vanadium redox flow batteries: a review oriented to fluid-dynamic optimization[J]. Energies, 2021, 14(1): 176. |
| 9 | Dehghani-Sanij A R, Tharumalingam E, Dusseault M B, et al. Study of energy storage systems and environmental challenges of batteries[J]. Renewable and Sustainable Energy Reviews, 2019, 104: 192-208. |
| 10 | Wang T, Fu J H, Zheng M L, et al. Dynamic control strategy for the electrolyte flow rate of vanadium redox flow batteries [J]. Appl Energy, 2018, 227: 613-623. |
| 11 | 夏力行, 刘昊, 刘琳, 等. 有机氧化还原液流电池的研究进展[J]. 电化学, 2018, 24(5): 466-487. |
| Xia Li-xing, Liu Hao, Liu Lin, et al. Recent progresses in organic redox flow batteries[J]. Journal of Electrochemistry, 2018, 24(5): 466-487. | |
| 12 | Chen Hai-sheng, Tan Chun-qing, Yang Wei, et al. Progress in electrical energy storage system: a critical review[J]. Progress in Natural Science, 2009, 19(3): 291-312. |
| 13 | 纪燕男, 徐谦, 秦立宇, 等.液流电池技术评价指标的发展及其应用[J]. 江苏大学学报: 自然科学版, 2019, 40(4): 404-410. |
| Ji Yan-nan, Xu Qian, Qin Li-yu, et al. Development and applications of technological evaluation criteria for redox flow batteries[J]. Journal of Jiangsu University(Natural Science Edition), 2019, 40(4): 404-410. | |
| 14 | You Dong-jiang, Zhang Hua-min, Sun Chen-xi, et al. Simulation of the self-discharge process in vanadium redox flow battery[J]. Journal of Power Sources, 2011, 196(3): 1578-1585. |
| 15 | Jung S, Choi B, Park S, et al. Computational study of effects of contact resistance on a large‐scale vanadium redox flow battery stack[J]. International Journal of Energy Research, 2019, 43(6): 2343-2360. |
| 16 | Kapoor M, Gautam R K, Ramani V K, et al. Predicting operational capacity of redox flow battery using a generalized empirical correlation derived from dimensional analysis[J]. Chemical Engineering Journal, 2019, 379: No.122300. |
| 17 | Kim K J, Park M S, Kim Y J, et al. A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries[J]. Journal of Materials Chemistry A, 2015, 3(33): 16913-16933. |
| 18 | 赵峰鸣, 闻刚, 孔丽瑶, 等. 氮化钛纳米管作为钒电池负极对Ⅴ(Ⅱ)/Ⅴ(Ⅲ)的电化学性能[J]. 无机化学学报, 2017, 33(3): 501-508. |
| Zhao Feng-ming, Wen Gang, Kong Li-yao, et al. Electrochemical performance of titanium nitride nanotubes as negative electrode in a static vanadium battery towards V(Ⅱ) /V(Ⅲ) redox couple[J]. Chinese Journal of Inorganic Chemistry, 2017, 33(3): 501-508. | |
| 19 | Knehr K W, Kumbur E C. Open circuit voltage of vanadium redox flow batteries: discrepancy between models and experiments[J]. Electrochemistry Communications, 2011, 13(4): 342-345. |
| 20 | Liu Bo, Zhang Yu-xia, Jiang Yun-hu, et al. High performance acid-base composite membranes from sulfonated polysulfone containing graphitic carbon nitride nanosheets for vanadium redox flow battery[J]. Journal of Membrane Science, 2019, 591: No.117332. |
| 21 | Wang Wei, Luo Qing-tao, Li Bin, et al. Recent progress in redox flow battery research and development[J]. Advanced Functional Materials, 2013, 23(8): 970-986. |
| 22 | Flox C, Skoumal M, Rubio-Garcia J, et al. Strategies for enhancing electrochemical activity of carbon-based electrodes for all-vanadium redox flow batteries[J]. Appl Energy, 2013, 109: 344-351. |
| 23 | Hu Guang-jian, Jing Ming-hua, Wang Da-wei, et al. A gradient bi-functional graphene-based modified electrode for vanadium redox flow batteries[J]. Energy Storage Mater, 2018, 13: 66-71. |
| 24 | Cheng Di-xuan, Li Yue-han, Zhang Jin-liang, et al. Recent advances in electrospun carbon fiber electrode for vanadium redox flow battery: properties, structures, and perspectives[J]. Carbon, 2020, 170: 527-542. |
| 25 | Zhou X L, Zeng Y K, Zhu X B, et al. A high-performance dual-scale porous electrode for vanadium redox flow batteries[J]. Journal of Power Sources, 2016, 325: 329-336. |
| 26 | Park S E, Yang S Y, Kim K J. Boron-functionalized carbon felt electrode for enhancing the electrochemical performance of vanadium redox flow batteries[J]. Applied Surface Science, 2021, 546: No.148941. |
| 27 | Aaron D S, Liu Q, Tang Z, et al. Dramatic performance gains in vanadium redox flow batteries through modified cell architecture[J]. Journal of Power Sources, 2012, 206: 450-453. |
| 28 | Youn C, Song S A, Kim K, et al. Effect of nitrogen functionalization of graphite felt electrode by ultrasonication on the electrochemical performance of vanadium redox flow battery[J]. Materials Chemistry and Physics, 2019, 237: No. 121873. |
| 29 | Xu Ze-yu, Xiao Wei, Zhang Kai-yue, et al. An advanced integrated electrode with micron- and nano-scale structures for vanadium redox flow battery[J]. Journal of Power Sources, 2020, 450: No.227686. |
| 30 | 马奇会. 全钒氧化还原液流电池石墨毡电极的改性研究[D]. 哈尔滨:哈尔滨工业大学海洋科学与技术学院, 2015. |
| Ma Qi-hui. Modification of graphite felt electrodes for vanadium redox flow battery[D]. Harbin: School of Marine Science and Technology, Harbin Institute of Technology, 2015. | |
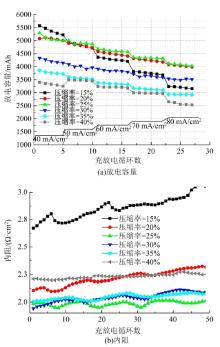
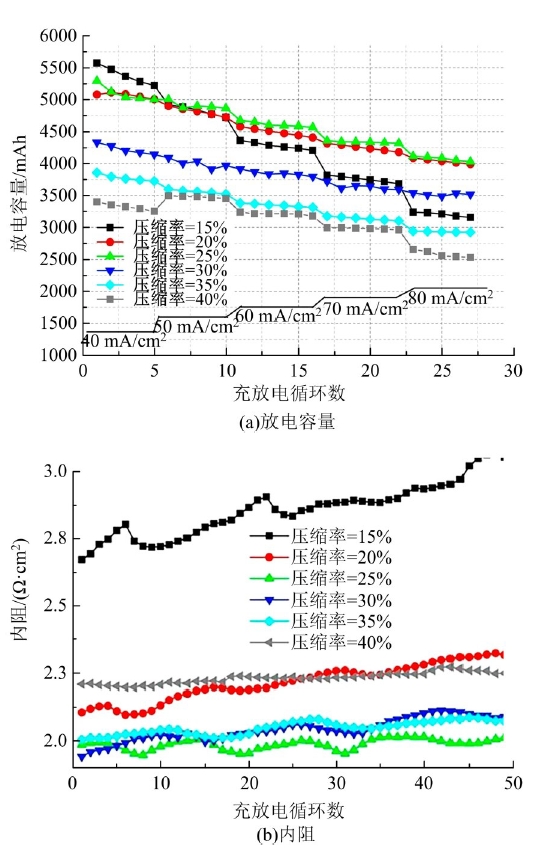
| 31 | Wu Lu-tao, Shen Yi, Yu Li-hong, et al. Boosting vanadium flow battery performance by Nitrogen-doped carbon nanospheres electrocatalyst[J]. Nano Energy, 2016, 28: 19-28. |
| 32 | Hassan A, Tzedakis T. Enhancement of the electrochemical activity of a commercial graphite felt for vanadium redox flow battery (VRFB), by chemical treatment with acidic solution of K2Cr2O7[J]. Journal of Energy Storage, 2019, 26: No.100967. |
| 33 | Chen Jian-zhang, Liao Wei-yang, Wen-yan Hsieh, et al. All-vanadium redox flow batteries with graphite felt electrodes treated by atmospheric pressure plasma jets[J]. Journal of Power Sources, 2015, 274: 894-898. |
| 34 | Kim S C, Lim H, Kim H, et al. Nitrogen and oxygen dual-doping on carbon electrodes by urea thermolysis and its electrocatalytic significance for vanadium redox flow battery[J]. Electrochimica Acta, 2020, 348: No.136286. |
| 35 | Pezeshki A M, Clement J T, Veith G M, et al. High performance electrodes in vanadium redox flow batteries through oxygen-enriched thermal activation[J]. Journal of Power Sources, 2015, 294: 333-338. |
| 36 | Vazquez-Galvan J, Flox C, Jervis J R, et al. High-power nitrided TiO2 carbon felt as the negative electrode for all-vanadium redox flow batteries[J]. Carbon, 2019, 148: 91-104. |
| 37 | Kil D, Lee H J, Park S, et al. Synthesis of activated graphite felts using short-term ozone/heat treatment for vanadium redox flow batteries[J]. Journal of the Electrochemical Society, 2017, 164(13):3011-3017. |
| 38 | Sun B, Skyllas-Kazacos M. ChemInform abstract: chemical modification of graphite electrode materials for vanadium redox flow battery application. part 2. acid treatments[J]. Chem Inform, 1992, 23(49): 18. |
| 39 | Yue Lu, Li Wei-shan, Sun Feng-qiang, et al. Highly hydroxylated carbon fibres as electrode materials of all-vanadium redox flow battery[J]. Carbon, 2010, 48(11): 3079-3090. |
| 40 | 杨春, 王树博, 谢晓峰, 等. 羟基自由基对全钒液流电池石墨毡电极的性能影响[J]. 化工学报, 2012, 63(): 188-193. |
| Yang Chun, Wang Shu-bo, Xie Xiao-feng, et al. Performance influence of hydroxyl radical on graphite felt electrode used in all vanadium redox flow battery[J]. CIESC Journal, 2012, 63(S1): 188-193. | |
| 41 | Flox C, Skoumal M, Rubio-Garcia J, et al. Strategies for enhancing electrochemical activity of carbon-based electrodes for all-vanadium redox flow batteries[J]. Appl Energy, 2013, 109: 344-351. |
| 42 | Cheng Di-xuan, Li Yue-hua, Han Chao, et al. Endowing electrospun carbon fiber with excellent electrocatalytic properties towards VO2+/VO2+ redox reaction for vanadium redox flow battery by in situ iridium decoration[J]. Physicochemical and Engineering Aspects, 2020, 586: No.124137. |
| 43 | Wang W H, Wang X D. Investigation of Ir-modified carbon felt as the positive electrode of an all-vanadium redox flow battery[J]. Electrochimica Acta, 2007, 52(24): 6755-6762. |
| 44 | Huang R H, Sun C H, Tseng T M, et al. Investigation of active electrodes modified with platinum/multiwalled carbon nanotube for vanadium redox flow battery[J]. Journal of the Electrochemical Society, 2012, 159(10): 1579-1586. |
| 45 | Li Bin, Gu Meng, Nie Zi-min, et al. Bismuth nanoparticle decorating graphite felt as a high-performance electrode for an all-vanadium redox flow battery[J]. Nano Letters, 2013, 13(3): 1330-1335. |
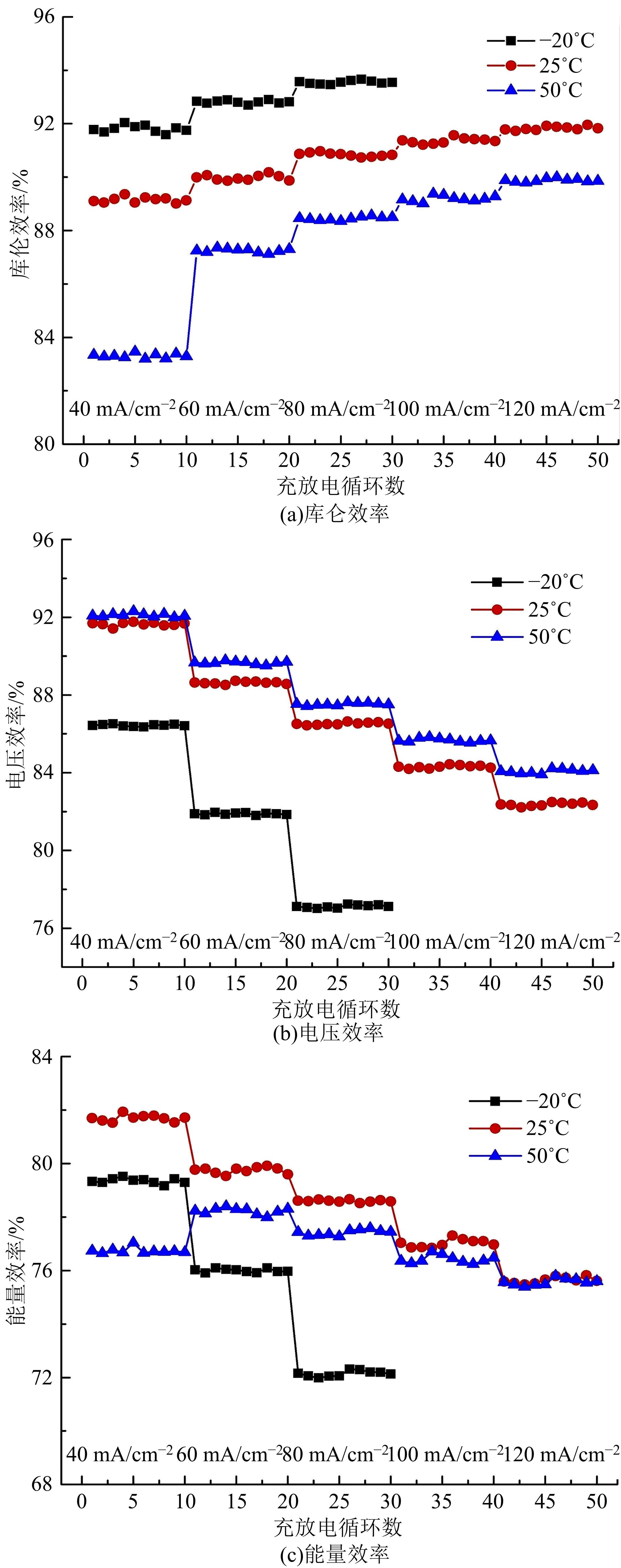
| 46 | He Zhang-xing, Dai Lei, Liu Su-qin, et al. Mn3O4 anchored on carbon nanotubes as an electrode reaction catalyst of V(IV)/V(V) couple for vanadium redox flow batteries[J]. Electrochimica Acta, 2015, 176: 1434-1440. |
| 47 | Wu Xiao-xin, Xu Hong-feng, Lu Lu, et al. PbO2-modified graphite felt as the positive electrode for an all-vanadium redox flow battery[J]. Journal of Power Sources, 2014, 250: 274-278. |
| 48 | Shen Yang, Xu Hong-feng, Xu Peng-cheng, et al. Electrochemical catalytic activity of tungsten trioxide- modified graphite felt toward VO2+/VO2+ redox reaction[J]. Electrochimica Acta, 2014, 132: 37-41. |
| 49 | Vazquez-Galvan J, Flox C, Fàbrega Cristian, et al. Hydrogen-treated rutile TiO2 shell in graphite-core structure as a negative electrode for high-performance vanadium redox flow batteries[J]. Chemsuschem, 2017, 10(9): 2089-2098. |
| 50 | Wang Q, Qu Z G, Jiang Z Y, et al. Numerical study on vanadium redox flow battery performance with non-uniformly compressed electrode and serpentine flow field[J]. Applied Energy, 2018, 220: 106-116. |
| 51 | Lu Meng-yue, Yang Wei-wei, Bai Xiao-shuai, et al. Performance improvement of a vanadium redox flow battery with asymmetric electrode designs[J]. Electrochim Acta, 2019, 319: 210-226. |
| 52 | Park S K, Shim J, Yang J H, et al. The influence of compressed carbon felt electrodes on the performance of a vanadium redox flow battery[J]. Electrochim Acta, 2014, 116: 447-452. |
| 53 | Oh K, Won S, Ju H. Numerical study of the effects of carbon felt electrode compression in all-vanadium redox flow batteries[J]. Electrochimica Acta, 2015, 181: 13-23. |
| 54 | Sang J Y, Kim S, Kim D K. Optimization of local porosity in the electrode as an advanced channel for all-vanadium redox flow battery[J]. Energy, 2019, 172: 26-35. |
| 55 | Ghimire P C, Arjun B, Schweiss R, et al. A comprehensive study of electrode compression effects in all vanadium redox flow batteries including locally resolved measurements[J]. Applied Energy, 2018, 230: 974-982. |
| 56 | Skyllas-Kazacos M, Kazacos G, Poon G, et al. Recent advances with UNSW vanadium-based redox flow batteries[J]. International Journal of Energy Research, 2010, 34(2): 182-189. |
| 57 | Lourenssen K, Williams J, Ahmadpour F, et al. Vanadium redox flow batteries: a comprehensive review[J]. Journal of Energy Storage, 2019, 25: No.00844. |
| 58 | Lourenssen K, Williams J, Ahmadpour F, et al. Design, development, and testing of a low-concentration vanadium redox flow battery[J]. Journal of Electrochemical Energy Conversion and Storage, 2021, 18(1): 1-17. |
| 59 | Poli N, Schaffer M, Trovo A, et al. Novel electrolyte rebalancing method for vanadium redox flow batteries[J]. Chemical Engineering Journal, 2021, 405: No.126583. |
| 60 | Li W, Zaffou R, Sholvin C C, et al. Vanadium redox-flow-battery electrolyte preparation with reducing agents[J]. ECS Transactions, 2013, 53(7): 93-99. |
| 61 | Dassisti M, Cozzolino G, Chimienti M, et al. Sustainability of vanadium redox-flow batteries: benchmarking electrolyte synthesis procedures[J]. International Journal of Hydrogen Energy, 2016, 41(37): 16477-16488. |
| 62 | Ngamsai K, Arpornwichanop A. Analysis and measurement of the electrolyte imbalance in a vanadium redox flow battery[J]. Journal of Power Sources, 2015, 282: 534-543. |
| 63 | Chang Fang, Hu Chang-wei, Liu Xiao-jiang, et al. Coulter dispersant as positive electrolyte additive for the vanadium redox flow battery[J]. Electrochim Acta, 2012, 60: 334-338. |
| 64 | Choi C, Kim S, Kim R, et al. A review of vanadium electrolytes for vanadium redox flow batteries[J]. Renewable and Sustainable Energy Reviews, 2017, 69: 263-274. |
| 65 | Wen Yue-hua, Xu Yan, Cheng Jie, et al. Investigation on the stability of electrolyte in vanadium flow batteries[J]. Electrochimica Acta, 2013, 96: 268-273. |
| 66 | álvaro Cunha, Brito F P, Martins J, et al. Assessment of the use of vanadium redox flow batteries for energy storage and fast charging of electric vehicles in gas stations[J]. Energy, 2016, 115: 1478-1494. |
| 67 | El Hage R, Chauvet F, Biscans B, et al. Kinetic study of the dissolution of vanadyl sulfate and vanadium pentoxide in sulfuric acid aqueous solution[J]. Chemical Engineering Science, 2019, 199: 123-136. |
| 68 | Wang Ke, Zhang Yun Nong, Liu Le, et al. Broad temperature adaptability of vanadium redox flow battery-part 3: the effects of total vanadium concentration and sulfuric acid concentration[J]. Electrochim Acta, 2018, 259: 11-19. |
| 69 | Xiao Shui-bo, Yu Li-hong, Wu Lan-tao, et al. Broad temperature adaptability of vanadium redox flow battery-part 1: electrolyte research[J]. Electrochim Acta, 2016, 187: 525-534. |
| 70 | Skyllas K M. Thermal stability of concentrated v(v) electrolytes in the vanadium redox cell[J]. Journal of the Electrochemical Society, 1996, 143(4): L86-L88. |
| 71 | Yang Ya-dong, Zhang Yi-min, Liu Tao, et al. Improved broad temperature adaptability and energy density of vanadium redox flow battery based on sulfate-chloride mixed acid by optimizing the concentration of electrolyte[J]. Journal of Power Sources, 2019, 415: 62-68. |
| 72 | Yang Ya-dong, Zhang Yi-min, Tang Li, et al. Investigations on physicochemical properties and electrochemical performance of sulfate-chloride mixed acid electrolyte for vanadium redox flow battery[J]. Journal of Power Sources, 2019, 434: No.226719. |
| 73 | 刘崇忠. 全钒液流电池电解液的研究[D]. 杭州:浙江工业大学化学工程学院, 2015. |
| Liu Chong-zhong. The research on the electrolyte of all vanadium redox flow battery[D]. Hangzhou: College of Chemical Engineering, Zhejiang University of Technology, 2015. | |
| 74 | Wang Q H, Daoud W A. Temperature influence on the reaction kinetics of V(IV)/V(V) in methanesulfonic acid for all-vanadium redox flow battery[J]. Electrochimica Acta, 2016, 214: 11-18. |
| 75 | Kausar N, Mousa A, Skyllas-Kazacos M. The effect of additives on the high-temperature stability of the vanadium redox flow battery positive electrolytes[J]. ChemElectroChem, 2016, 3(2): 276-282. |
| 76 | Ding Cong, Ni Xiao, Li Xian-feng, et al. Effects of phosphate additives on the stability of positive electrolytes for vanadium flow batteries[J]. Electrochim Acta, 2015, 164: 307-314. |
| 77 | Roznyatovskaya N V, Roznyatovsky V A, Hohne C C, et al. The role of phosphate additive in stabilization of sulphuric-acid-based vanadium(V) electrolyte for all-vanadium redox-flow batteries[J]. Journal of Power Sources, 2017, 363: 234-243. |
| 78 | 陈孝娥, 崔旭梅, 曾志勇, 等. NaCl作为添加剂对钒电池正极电解液性能的影响[J]. 电源技术, 2018, 42(6): 840-842. |
| Chen Xiao-e, Cui Xu-mei, Zeng Zhi-yong, et al. Effect of NaCl as additive for positive electrolyte on the properties of vanadium redox flow battery[J]. Chinese Journal of Power Sources, 2018, 42(6): 840-842. | |
| 79 | Nguyen T D, Wang L P, Whitehead A, et al. Insights into the synergistic effect of ammonium and phosphate-containing additives for a thermally stable vanadium redox flow battery electrolyte[J]. Journal of Power Sources, 2018, 402: 75-81. |
| 80 | Wei Xian-li, Liu Su-qin, Wang Jue, et al. Boosting the performance of positive electrolyte for VRFB by employing zwitterion molecule containing sulfonic and pyridine groups as the additive[J]. Ionics, 2020, 26(6): 3147-3159. |
| 81 | Cao Liu-yue, Skyllas-Kazacos M, Menictas C, et al. A review of electrolyte additives and impurities in vanadium redox flow batteries[J]. Journal of Energy Chemistry, 2018, 27(5): 1269-1291. |
| 82 | Wu Xiao-jun, Liu Su-qin, Wang Nan-fang, et al. Influence of organic additives on electrochemical properties of the positive electrolyte for all-vanadium redox flow battery[J]. Electrochim Acta, 2012, 78: 475-482. |
| 83 | Hwang J, Kim B M, Moon J, et al. A highly efficient and stable organic additive for the positive electrolyte in vanadium redox flow batteries: taurine biomolecules containing -NH2 and -SO3H functional groups[J]. Journal of Materials Chemistry A, 2018, 6(11): 4695-4705. |
| 84 | Abbas S, Hwang J, Kim H, et al. Enzyme-inspired formulation of the electrolyte for stable and efficient vanadium redox flow batteries at high temperatures[J]. ACS Appl Mater Interfaces, 2019, 11(30): 26842-26853. |
| 85 | Tang Ao, Bao Jie, Skyllas-Kazacos M. Dynamic modelling of the effects of ion diffusion and side reactions on the capacity loss for vanadium redox flow battery[J]. Journal of Power Sources, 2011, 196(24): 10737-10747. |
| 86 | Jirabovornwisut T, Arpornwichanop A. A review on the electrolyte imbalance in vanadium redox flow batteries[J]. International Journal of Hydrogen Energy, 2019, 44(45): 24485-24509. |
| 87 | Ngamsai K, Arpornwichanop A. Investigating the air oxidation of V(II) ions in a vanadium redox flow battery[J]. Journal of Power Sources, 2015, 295: 292-298. |
| 88 | Xu Wen-jie, Long Jun, Liu Jun, et al. Novel highly efficient branched polyfluoro sulfonated polyimide membranes for application in vanadium redox flow battery[J]. Journal of Power Sources, 2021, 485: No. 229354. |
| 89 | Xi Jing-yu, Wu Zeng-hua, Teng Xiang-guo, et al. Self-assembled polyelectrolyte multilayer modified Nafion membrane with suppressed vanadium ion crossover for vanadium redox flow batteries[J]. Journal of Materials Chemistry, 2008, 18(11): 1232-1238. |
| 90 | Kraytsberg A, Ein-Eli Y. Review of advanced materials for proton exchange membrane fuel cells[J]. Energy & Fuels, 2017, 28(12): 7303-7330. |
| 91 | Ye Jia-jie, Yuan Du, Ding Mei, et al. A cost-effective nafion/lignin composite membrane with low vanadium ion permeation for high performance vanadium redox flow battery[J]. Journal of Power Sources, 2021, 482: No. 229023. |
| 92 | Minke C, Turek T. Economics of vanadium redox flow battery membranes[J]. Journal of Power Sources, 2015, 286: 247-257. |
| 93 | Wang Tong-shuai, Moon S J, Hwang D S, et al. Selective ion transport for a vanadium redox flow battery (VRFB) in nano-crack regulated proton exchange membranes[J]. Journal of Membrane Science, 2019, 583: 16-22. |
| 94 | Yang Rui-dong, Cao Zi-shu, Yang Shao-wei, et al. Colloidal silicalite-nafion composite ion exchange membrane for vanadium redox-flow battery[J]. Journal of Membrane Science, 2015, 484: 1-9. |
| 95 | Zhang Yang, Cui Zhi-ming, Zhao Cheng-ji, et al. Synthesis and characterization of novel sulfonated poly(arylene ether ketone) copolymers with pendant carboxylic acid groups for proton exchange membranes[J]. Journal of Power Sources, 2009, 191(2): 253⁃258. |
| 96 | Ji Y, Tay Z Y, Li S F Y. Highly selective sulfonated poly(ether ether ketone)/titanium oxide composite membranes for vanadium redox flow batteries[J]. Journal of Membrane Science, 2017, 539: 197-205. |
| 97 | Wang T S, Jeon J Y, Han J, et al. Poly(terphenylene) anion exchange membranes with high conductivity and low vanadium permeability for vanadium redox flow batteries (VRFBs)[J]. J Membr Sci, 2020, 598: No.117665. |
| 98 | Yuan Zhi-zhang, Duan Yin-qi, Zhang Hong-zhang, et al. Advanced porous membranes with ultra-high selectivity and stability for vanadium flow batteries[J]. Energy & Environmental Science, 2016, 9(2): 441-447. |
| 99 | Ding Li-ming, Song Xi-peng, Wang Li-hua, et al. Preparation of dense polybenzimidazole proton exchange membranes with different basicity and flexibility for vanadium redox flow battery applications[J]. Electrochim Acta, 2018, 292: 10-19. |
| 100 | Jung M, Lee W, Krishnan N N, et al. Porous-Nafion/PBI composite membranes and Nafion/PBI blend membranes for vanadium redox flow batteries[J]. Applied Surface Science, 2018, 450: 301-311. |
| 101 | Hwang G J, Kim S W, In D M, et al. Application of the commercial ion exchange membranes in the all-vanadium redox flow battery[J]. Journal of Industrial and Engineering Chemistry, 2018, 60: 360-365. |
| 102 | Park E J, Maurya S, Martinez U, et al. Quaternized poly(arylene ether benzonitrile) membranes for vanadium redox flow batteries[J]. Journal of Membrane Science, 2021, 617: No.118565. |
| 103 | Chen D Y, Hickner M A, Agar E, et al. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries[J]. Electrochemistry Communications, 2013, 26: 37-40. |
| 104 | Dai Ji-cui, Dong Yi-chao, Gao Peng, et al. A sandwiched bipolar membrane for all vanadium redox flow battery with high coulombic efficiency[J]. Polymer, 2018, 140: 233-239. |
| 105 | Xia Zi-jun, Ying Li-bin, Fang Jian-hua, et al. Preparation of covalently cross-linked sulfonated polybenzimidazole membranes for vanadium redox flow battery applications[J]. Journal of Membrane Science, 2017, 525: 229-239. |
| 106 | Wang Gang, Zhang Miao-miao, He Zhen-jia, et al. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone) with ammonium polyphosphate for vanadium redox flow battery applications[J]. Journal of Applied Polymer Science, 2021, 138(25): No. 50592. |
| 107 | 李玲. 季铵功能化侧链型阴离子交换膜的制备[D]. 厦门:厦门大学化学化工学院, 2019. |
| Li Ling. Preparation of quaternary ammoinium functionalized side chain anion exchange membranes[D]. Xiamen: College of Chemistry and Chemical Engineering, Xiamen University, 2019. | |
| 108 | Wang Lie, Yu Li-hong, Mu Di, et al. Acid-base membranes of imidazole-based sulfonated polyimides for vanadium flow batteries[J]. Journal of Membrane Science, 2018, 552: 167-176. |
| 109 | 陈宇宁, 张守海, 蹇锡高. 钒电池用两性离子交换膜的研究进展[J]. 膜科学与技术, 2020, 40(3): 151-160. |
| Chen Yu-ning, Zhang Shou-hai, Jian Xi-gao. Recent progress on amphoteric ion exchange membranes for vanadium redox flow battery applications[J]. Membrane Science and Technology, 2020, 40(3): 151-160. | |
| 110 | Yan Xiao-ming, Zhang Cai-mian, Dai Yan, et al. A novel imidazolium-based amphoteric membrane for high-performance vanadium redox flow battery[J]. Journal of Membrane Science, 2017, 544: 98-107. |
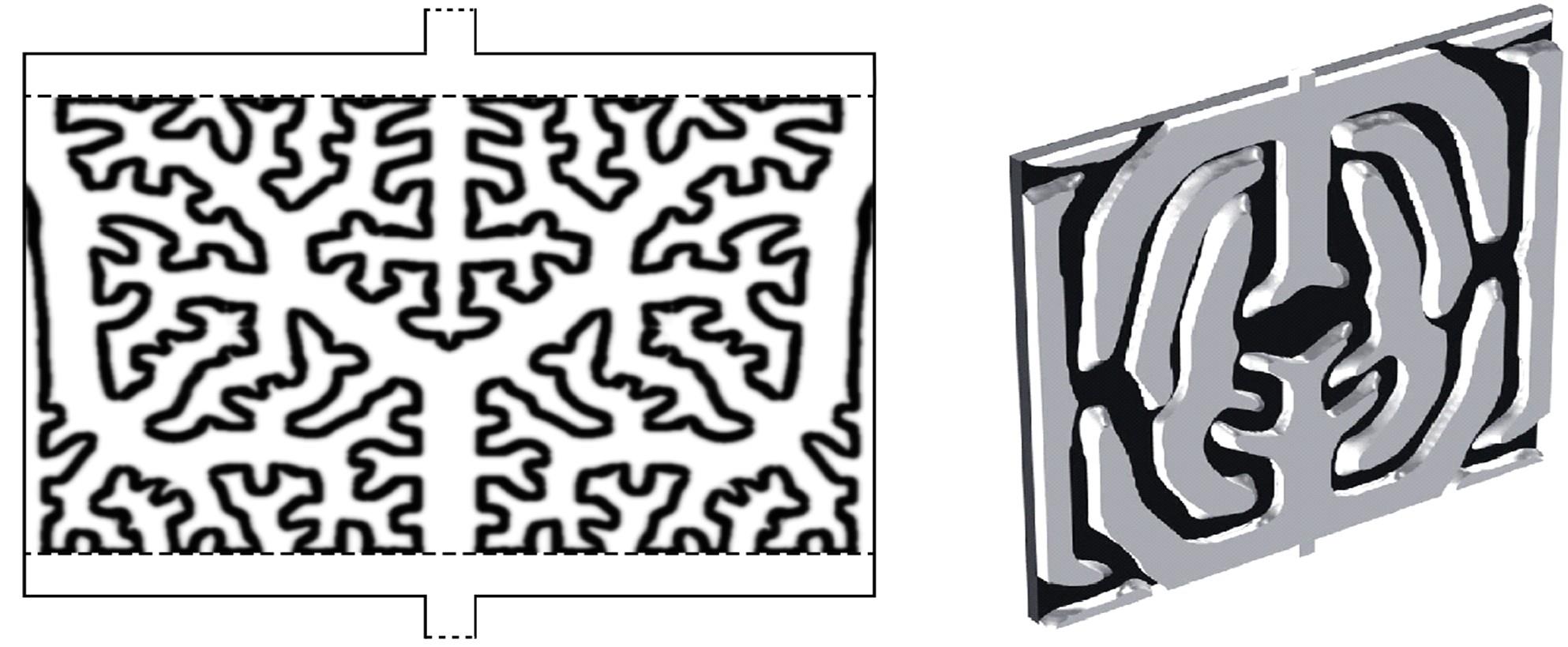
| 111 | Xi Xiao-li, Ding-cong, Zhang Hong-zhang, et al. Solvent responsive silica composite nanofiltration membrane with controlled pores and improved ion selectivity for vanadium flow battery application[J]. Journal of Power Sources, 2015, 274: 1126-1134. |
| 112 | Chen Dong-ju, Li Dan-dan, Li Xian-feng. Hierarchical porous poly (ether sulfone) membranes with excellent capacity retention for vanadium flow battery application[J]. Journal of Power Sources, 2017, 353: 11-18. |
| 113 | Zhang Hong-zhang, Zhang H, Li X, et al. Nanofiltration (NF) membranes: the next generation separators for all vanadium redox flow batteries (VRBs)? [J]. Energy and Environmental Science, 2011, 4(5): 1676-1679. |
| 114 | Zhou X L, Zhao T S, An L, et al. Modeling of ion transport through a porous separator in vanadium redox flow batteries[J]. Journal of Power Sources, 2016, 327: 67-76. |
| 115 | Zhang Hong-zhang, Zhang Hua-min, Li Xian-feng, et al. Silica modified nanofiltration membranes with improved selectivity for redox flow battery application[J]. Energy and Environmental Science, 2012, 5(4): 6299-6303. |
| 116 | Sun Chen-xi, Chen Jian, Zhang Hua-min, et al. Investigations on transfer of water and vanadium ions across Nafion membrane in an operating vanadium redox flow battery[J]. Journal of Power Sources, 2010, 195(3): 890-897. |
| 117 | Kumar S, Jayanti S. Effect of flow field on the performance of an all-vanadium redox flow battery[J]. Journal of Power Sources, 2016, 307: 782-787. |
| 118 | Ulaganathan M, Aravindan V, Yan Q Y, et al. Recent advancements in all-vanadium redox flow batteries[J]. Advanced Materials Interfaces, 2016, 3(1): No.1500309. |
| 119 | Tang A, Bao J, Skyllas-Kazacos M. Dynamic modelling of the effects of ion diffusion and side reactions on the capacity loss for vanadium redox flow battery[J]. Journal of Power Sources, 2011, 196(24): 10737-10747. |
| 120 | Mohammadi T, Mahdifar M, Chieng S C. Water transport study across ion exchange membranes in the vanadium redox flow battery[J]. Journal of Membrane Science, 1997, 133(2): 151-159. |
| 121 | Sepehr F, Paddison S J. The solvation structure and thermodynamics of aqueous vanadium cations[J]. Chem Phys Lett, 2013, 585: 53-58. |
| 122 | Messaggi M, Canzi P, Mereu R, et al. Analysis of flow field design on vanadium redox flow battery performance: development of 3D computational fluid dynamic model and experimental validation[J]. Appl Energy, 2018, 228: 1057-1070. |
| 123 | Yin Cong, Gao Yan, Xie Guang-you, et al. Three dimensional multi-physical modeling study of interdigitated flow field in porous electrode for vanadium redox flow battery[J]. Journal of Power Sources, 2019, 438: No.227023. |
| 124 | Zhang B W, Lei Y, Bai B F, et al. A two-dimensional model for the design of flow fields in vanadium redox flow batteries[J]. International Journal of Heat and Mass Transfer, 2019, 135: 460-469. |
| 125 | Lee J, Kim J, Park H. Numerical simulation of the power-based efficiency in vanadium redox flow battery with different serpentine channel size[J]. International Journal of Hydrogen Energy, 2019, 44(56): 29483-29492. |
| 126 | Ali E, Kwon H, Choi J, et al. A numerical study of electrode thickness and porosity effects in all vanadium redox flow batteries[J]. Journal of Energy Storage, 2020, 28: No.101208. |
| 127 | Houser J, Clement J, Pezeshki A, et al. Influence of architecture and material properties on vanadium redox flow battery performance[J]. Journal of Energy Storage, 2016, 302: 369-377. |
| 128 | Messaggi M, Rabissi C, Gambaro C, et al. Investigation of vanadium redox flow batteries performance through locally-resolved polarisation curves and impedance spectroscopy: insight into the effects of electrolyte, flow field geometry and electrode thickness[J]. Journal of Power Sources, 2020, 449: No.227588. |
| 129 | Xu Q, Zhao T S, Leung P K. Numerical investigations of flow field designs for vanadium redox flow batteries[J]. Appl Energy, 2013, 105: 47-56. |
| 130 | Maurya S, Nguyen P T, Kim Y S, et al. Effect of flow field geometry on operating current density, capacity and performance of vanadium redox flow battery[J]. Journal of Power Sources, 2018, 404: 20-27. |
| 131 | Macdonald M, Darling R M. Comparing velocities and pressures in redox flow batteries with interdigitated and serpentine channels[J]. Aiche Journal, 2019, 65(5):No. e16553. |
| 132 | Houser J, Clement J, Pezeshki A, et al. Influence of architecture and material properties on vanadium redox flow battery performance[J]. Journal of Power Sources, 2016, 302: 369-377. |
| 133 | Dennison C R, Agar E, Akuzum B, et al. Enhancing mass transport in redox flow batteries by tailoring flow field and electrode design[J]. Journal of the Electrochemical Society A, 2016, 163(1): 5163-5169. |
| 134 | Sun Jie, Zheng Meng-lian, Yang Zhong-shu, et al. Flow field design pathways from lab-scale toward large-scale flow batteries[J]. Energy, 2019, 173: 637-646. |
| 135 | Sun Jie, Zheng Meng-lian, Luo Yan-song, et al. Three-dimensional detached serpentine flow field design for redox flow batteries[J]. Journal of Power Sources, 2019, 428: 136-145. |
| 136 | Yaji K, Yamasaki S, Tsushima S, et al. Topology optimization for the design of flow fields in a redox flow battery[J]. Structural and Multidisciplinary Optimization, 2018, 57(2): 535-546. |
| 137 | Chen C H, Yaji K, Yamasaki S, et al. Computational design of flow fields for vanadium redox flow batteries via topology optimization[J]. The Journal of Energy Storage, 2019, 26: No.100990. |
| [1] | 刘宁庆, 韩雪, 张文彬. 基于混沌搜索的蜂窝网基站能量效率与服务质量的联合优化[J]. 吉林大学学报(工学版), 2016, 46(5): 1660-1666. |
| [2] | 李钊, 李培凤, 蔡沈锦. 基于动态中继激励的协作下行传输用户调度[J]. 吉林大学学报(工学版), 2016, 46(4): 1313-1319. |
| [3] | 张瑞华, 程合友, 贾智平. 基于能量效率的无线传感器网络分簇算法[J]. 吉林大学学报(工学版), 2010, 40(06): 1663-1667. |
| [4] | 薛建彬,朱延峰,袁占亭 . 一种适配数据速率的IEEE 802.16e休眠机制算法[J]. 吉林大学学报(工学版), 2009, 39(02): 519-0524. |
| [5] | 张瑞华,贾智平,袁东风 . 异构型无线传感器网络的生命周期[J]. 吉林大学学报(工学版), 2008, 38(05): 1136-1140. |
|
||