吉林大学学报(工学版) ›› 2024, Vol. 54 ›› Issue (1): 114-123.doi: 10.13229/j.cnki.jdxbgxb.20220218
• 材料科学与工程 • 上一篇
铝镓铟锡合金水解产氢速率的调控方法
- 1.吉林大学 材料科学与工程学院,长春 130022
2.吉林大学 汽车仿真与控制国家重点实验室,长春 130022
Control method on hydrogen production rate of aluminum gallium indium tin alloy
Qian GAO1,2( ),Dong-han LI1,Zhi-jiang JIN1,Jie SHI1
),Dong-han LI1,Zhi-jiang JIN1,Jie SHI1
- 1.College of Materials Science and Engineering,Jilin University,Changchun 130022,China
2.State Key Laboratory of Automotive Simulation and Control,Jilin University,Changchun 130022,China
摘要:
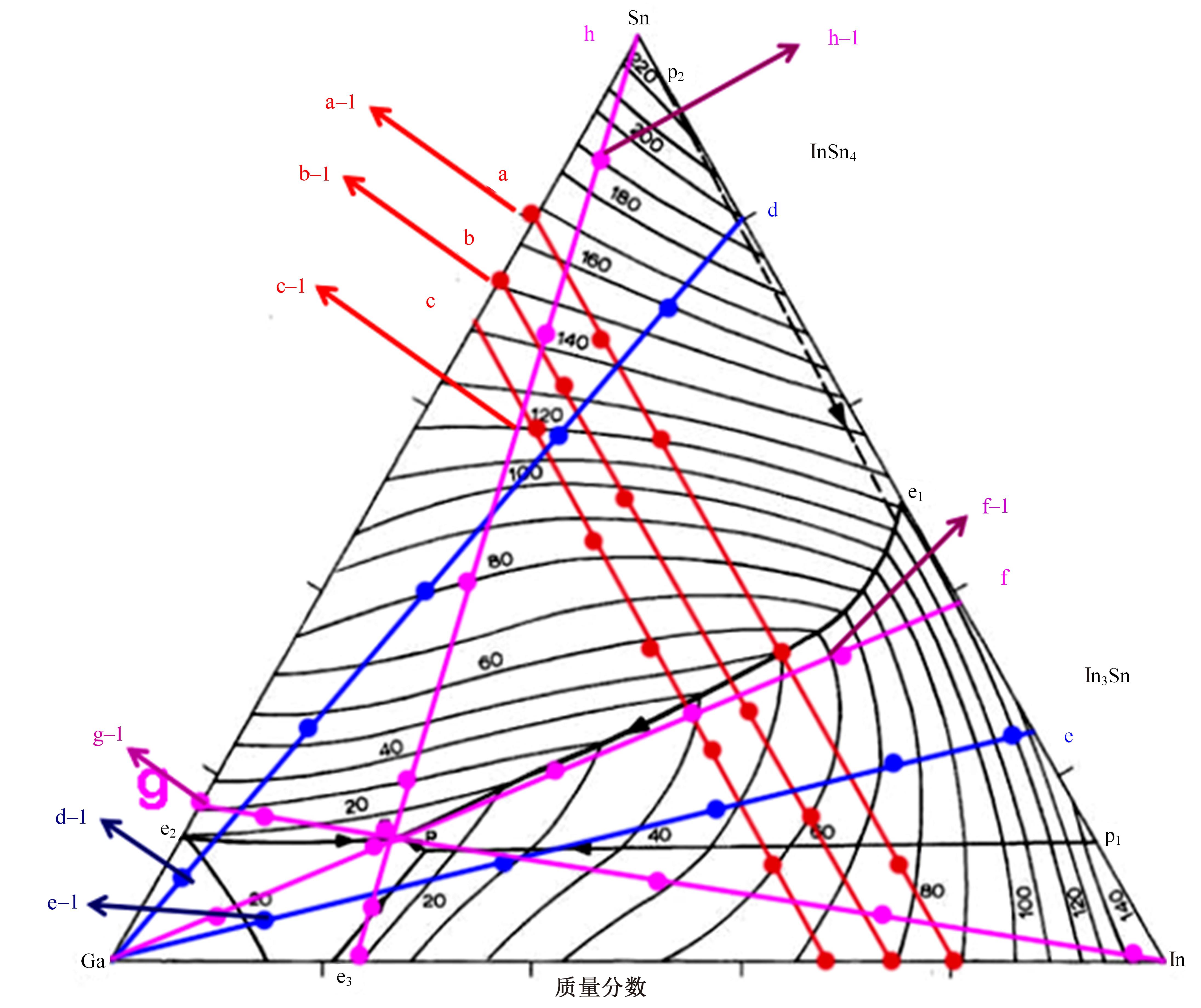
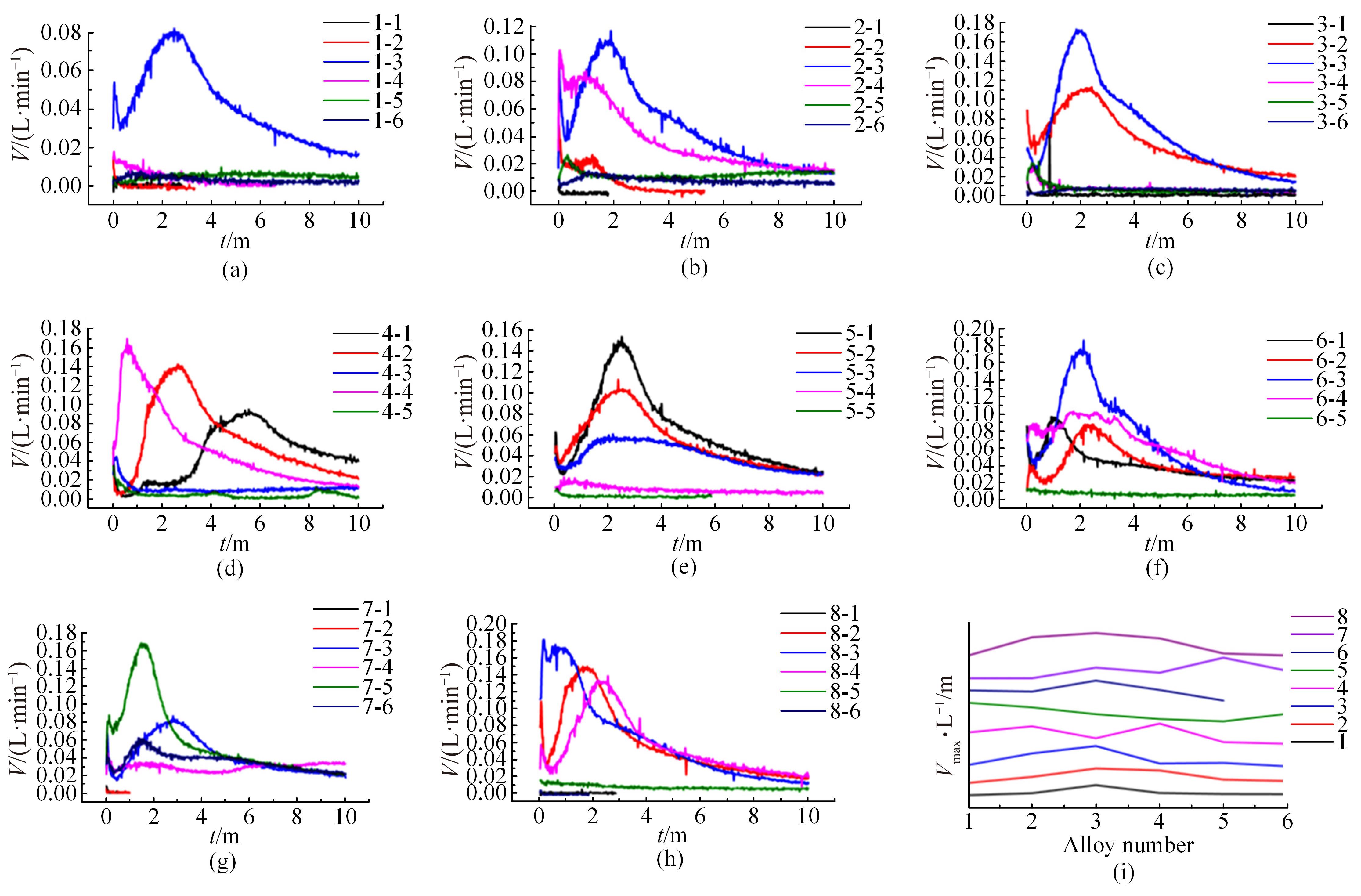
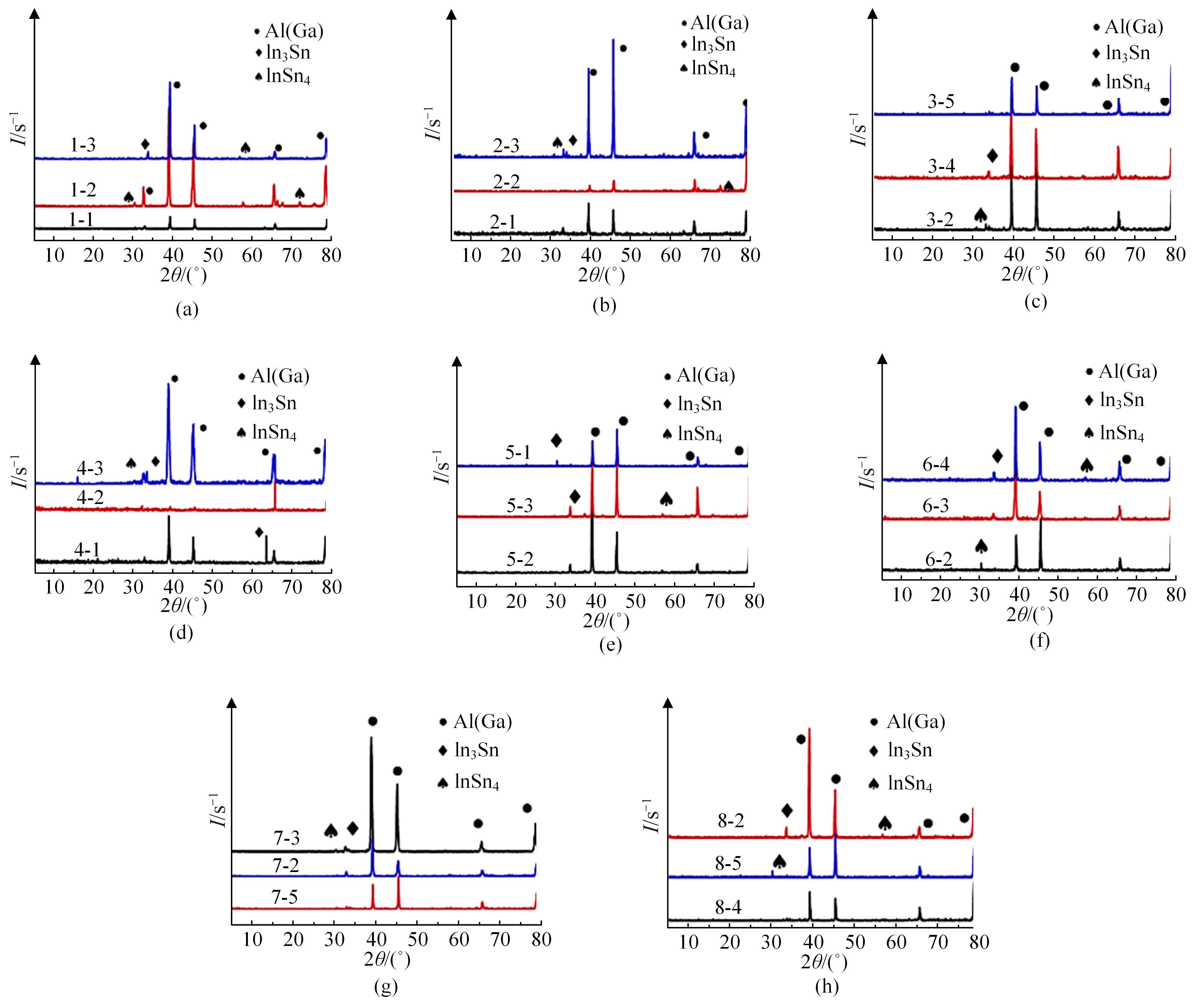
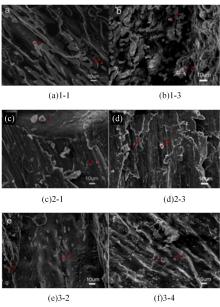
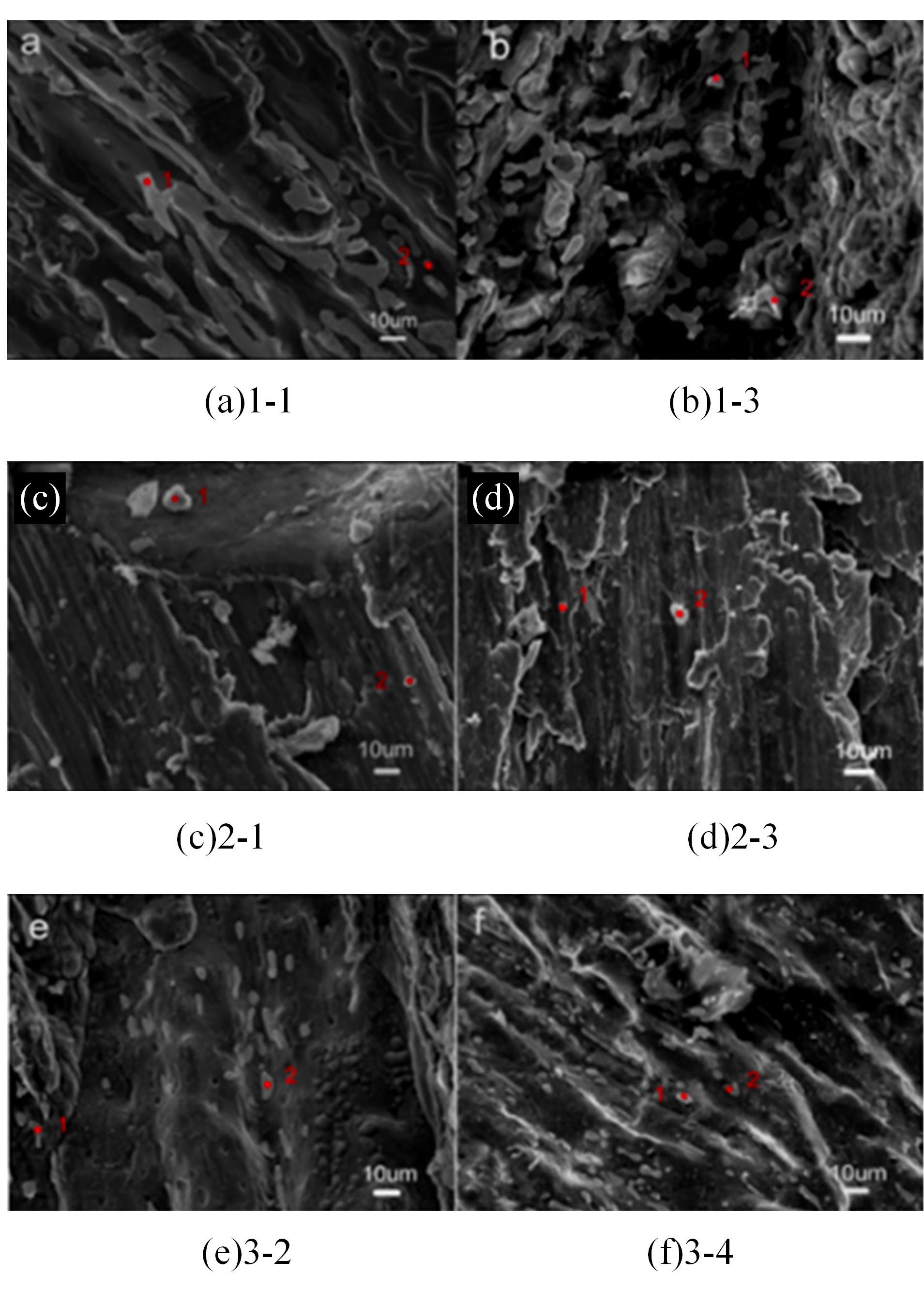
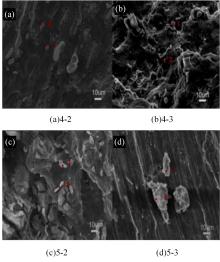
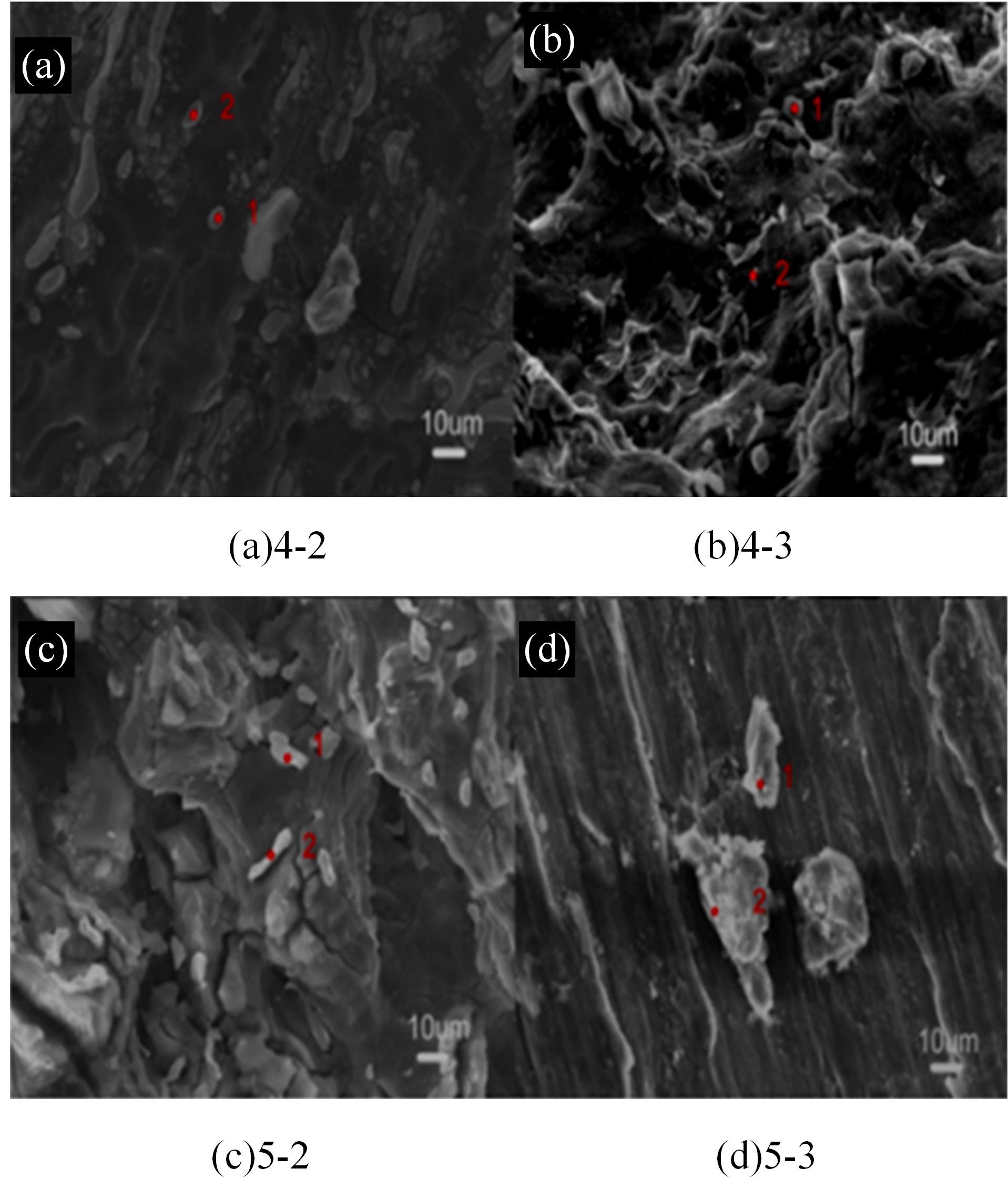
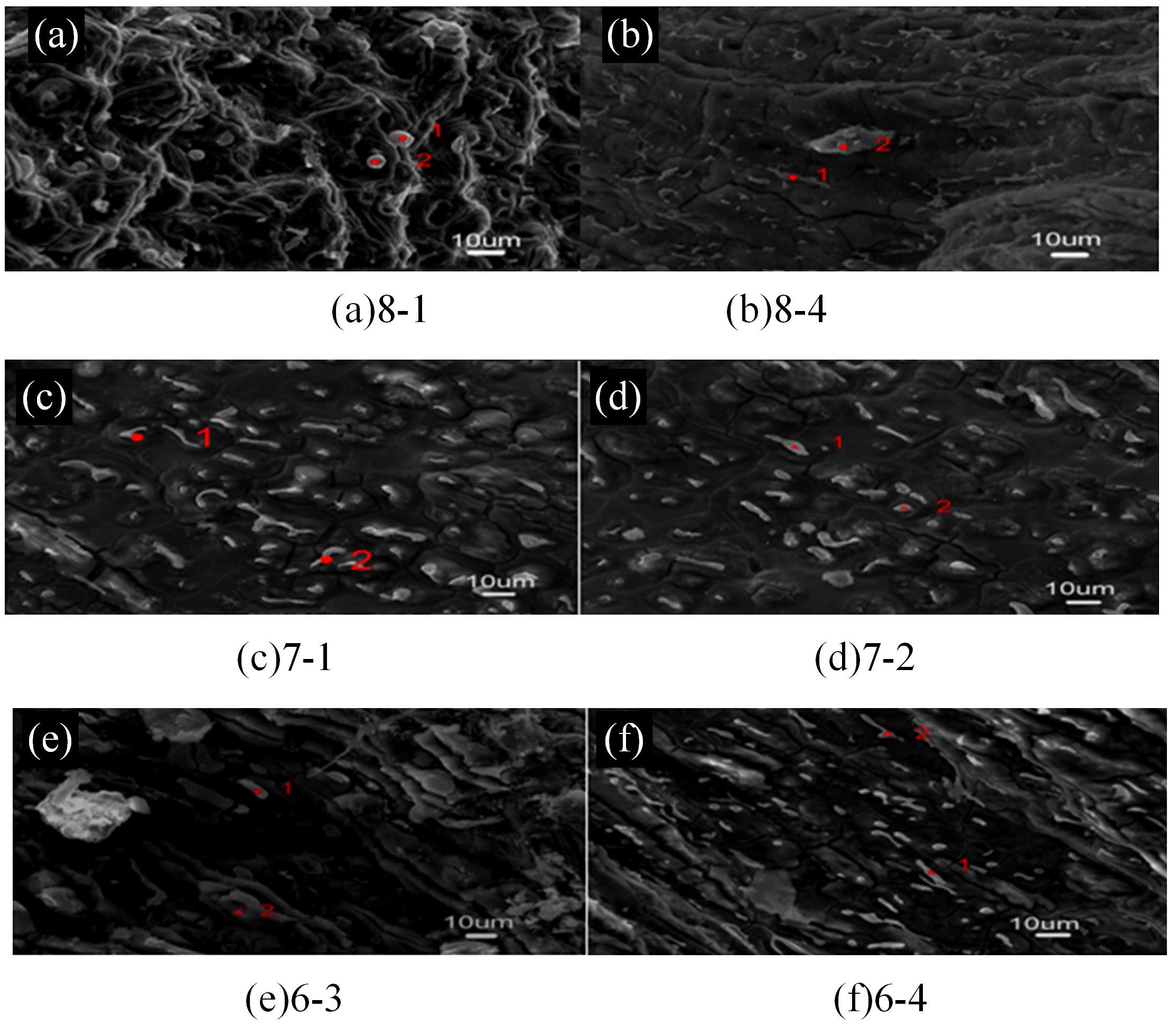
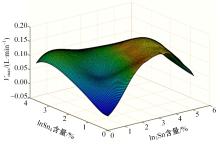
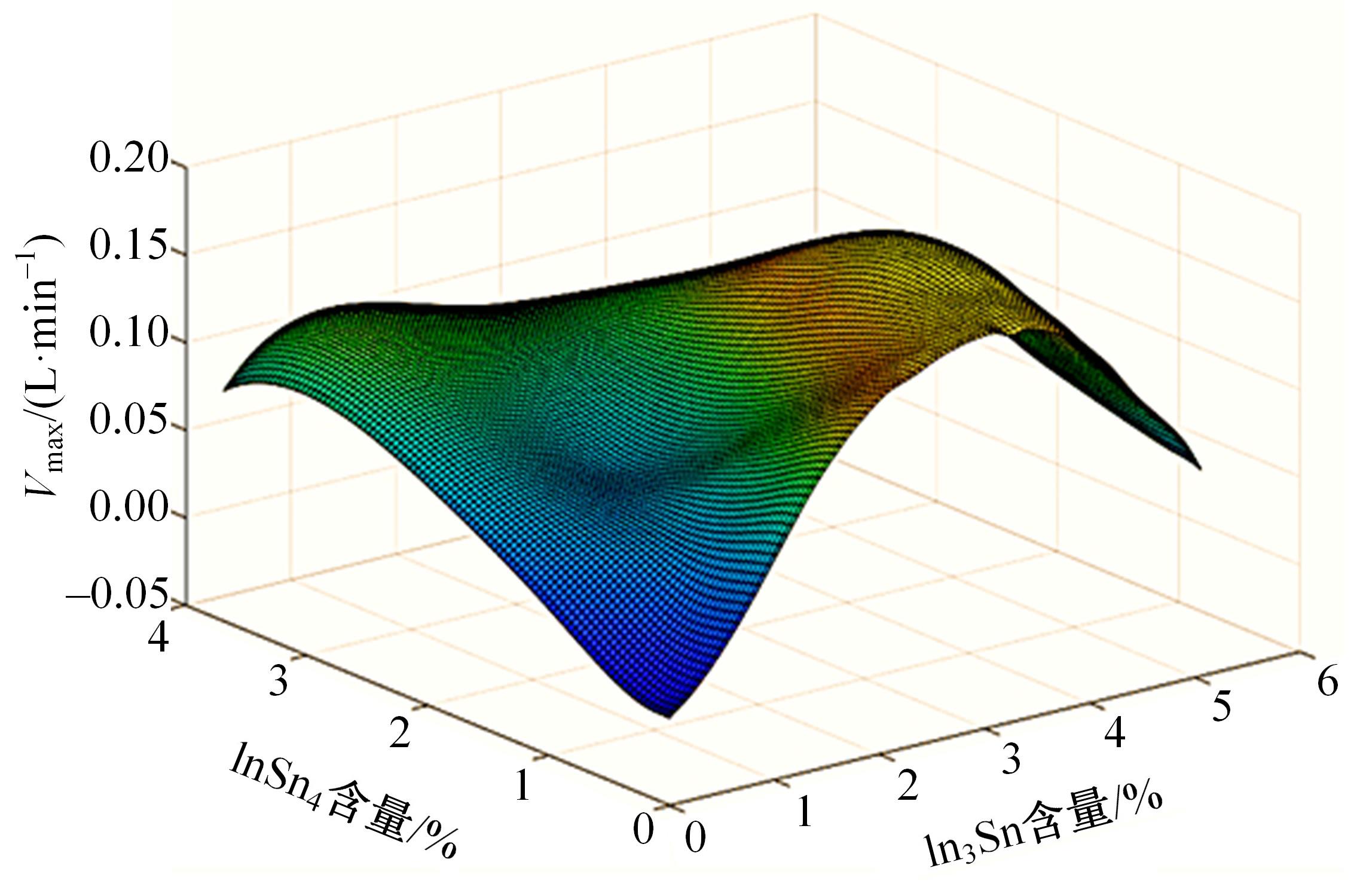
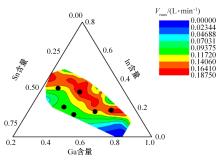
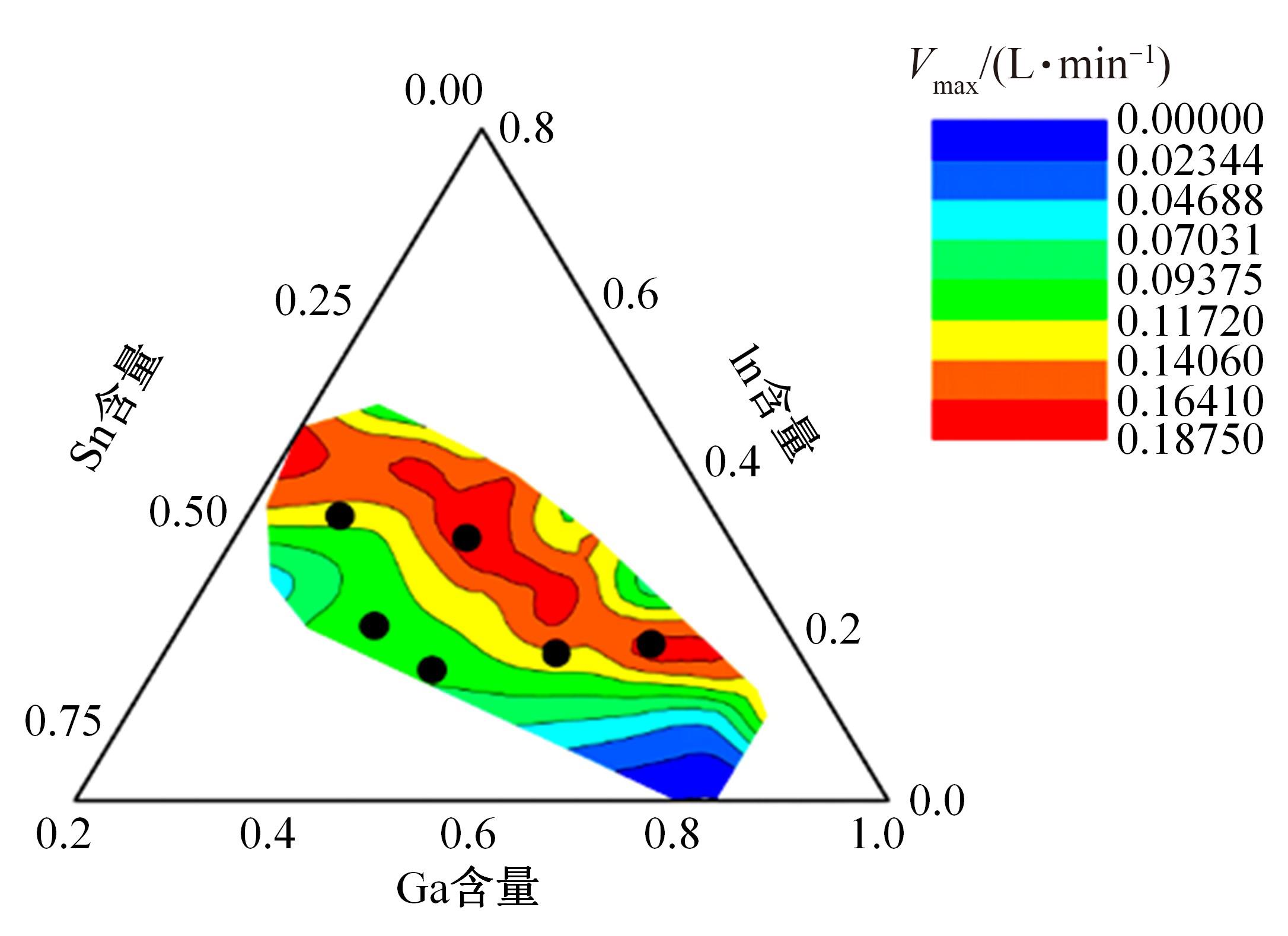
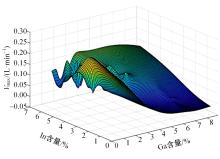
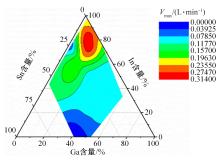
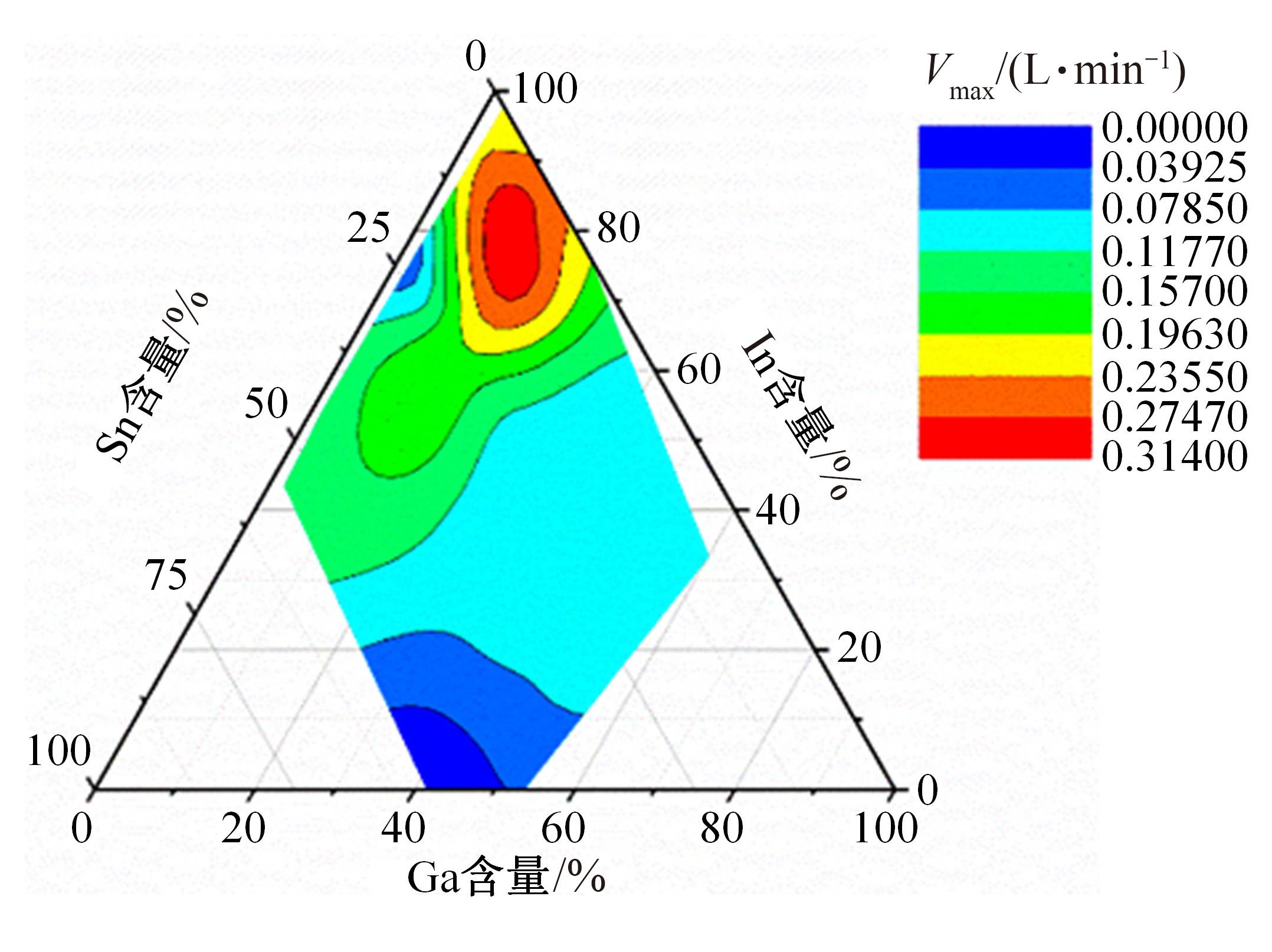
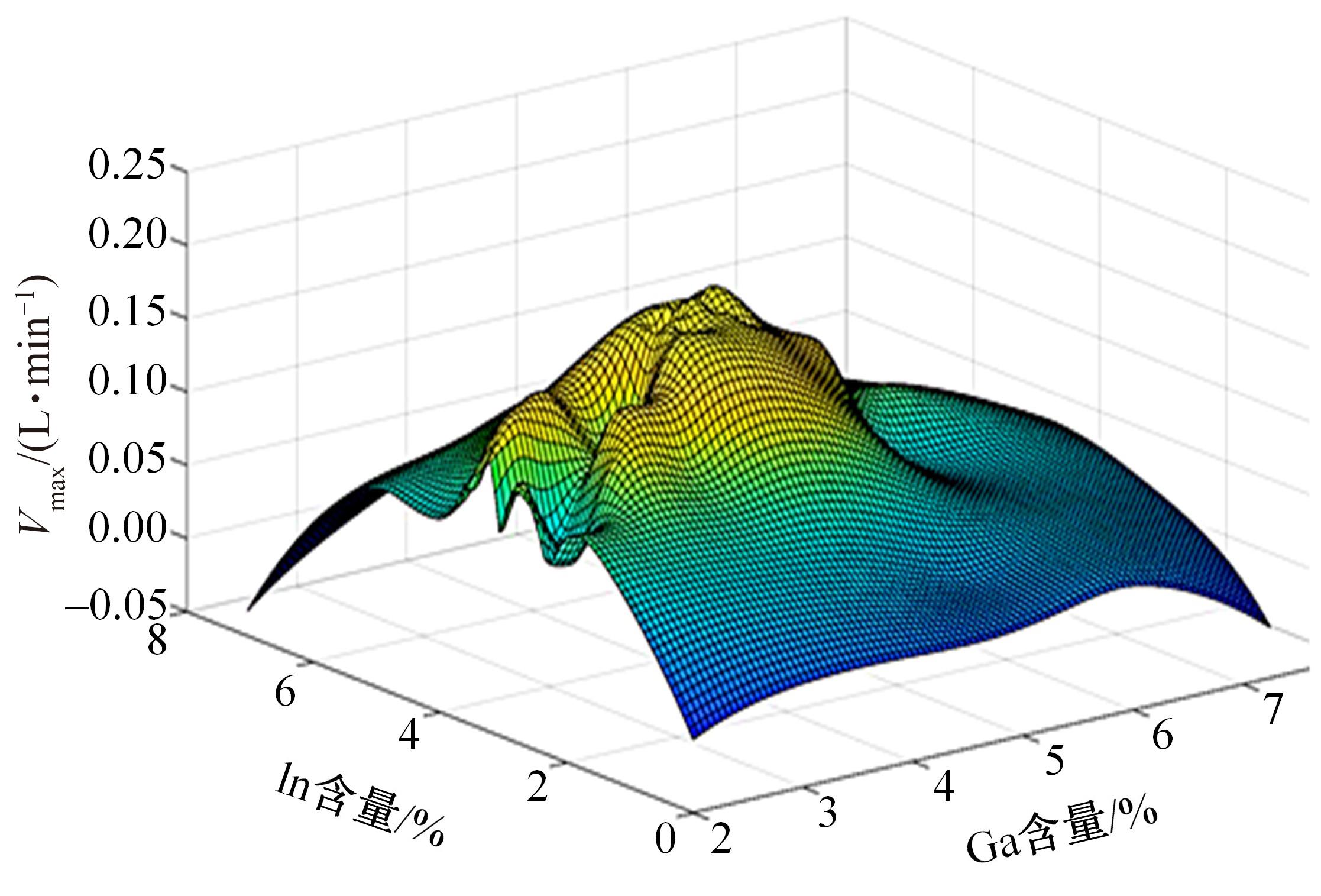
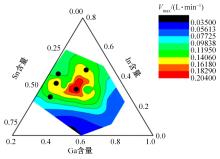
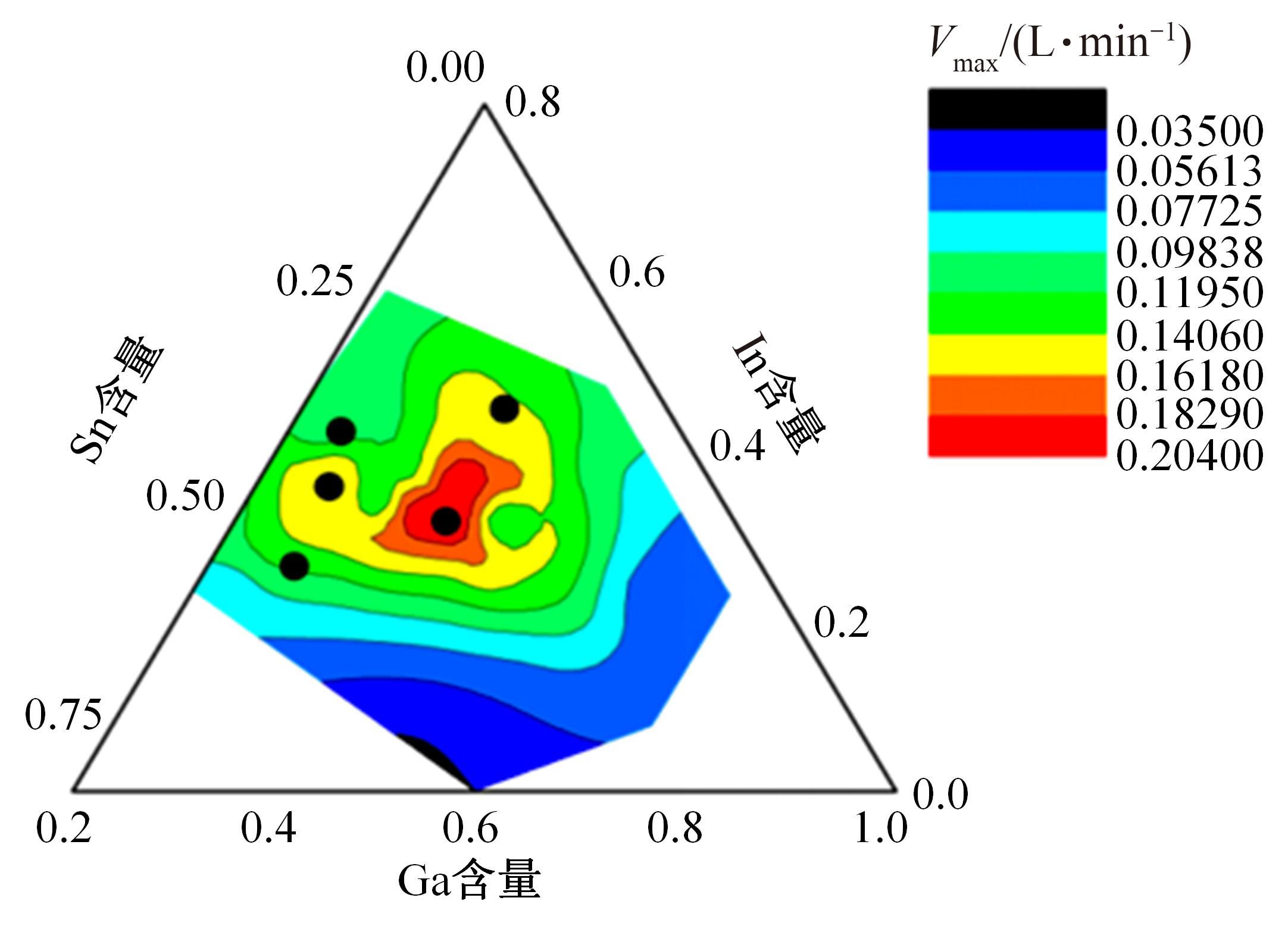
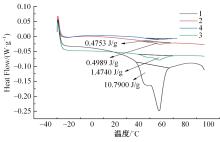
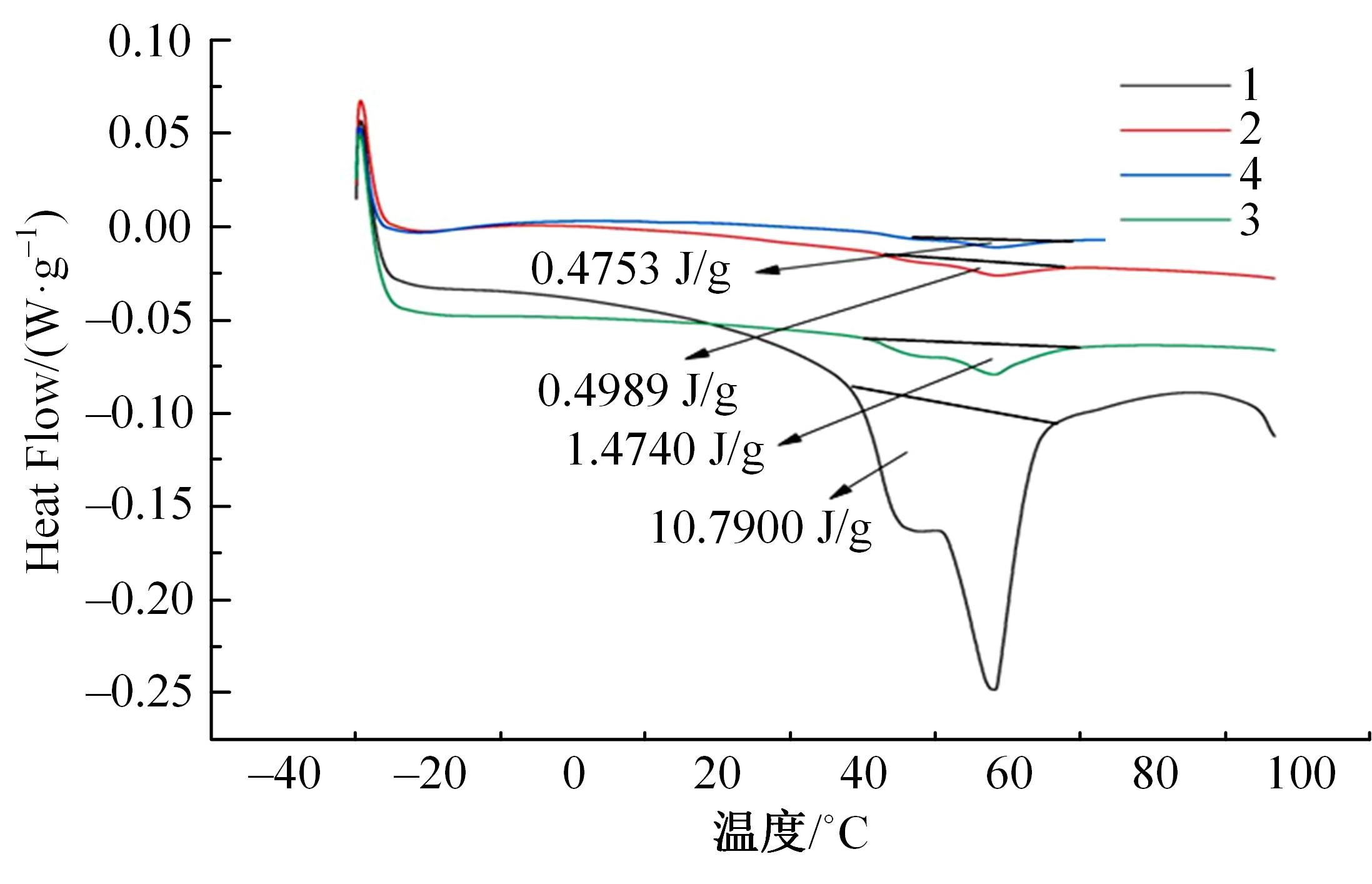
针对目前合金元素对铝水解制氢的活化机制尚不明晰,无法用来指导材料的配方设计与性能调控的问题,基于已有的实验经验结论和理论相图,进行了材料样本点的设计、合成、表征及产氢性能测试,并以合金速率峰值作为优化目标,分别探究了合金的物相组成和元素组成对产氢速率的耦合关系。结果显示:合金的元素组成与产氢峰值的耦合关系比物相组成的耦合关系具有更高的可信度,运用此耦合关系可以在一定程度上指导材料的定向设计,为未来此类材料的定向设计合成提供了一种新的思路。
中图分类号:
- TS912.3
| 1 | Berger M, Goldfarb J L. Understanding our energy footprint: undergraduate chemistry laboratory investigation of environmental impacts of solid fossil fuel wastes[J]. Journal of Chemical Education, 2017, 94(8): 1124-1128. |
| 2 | Europe Oil-Telegram Group. Statistical review of world energy 2016[J]. Europe Oil-Telegram, 2016,54(48/49): 9-11. |
| 3 | Xu S, Zhao X, Liu J. Liquid metal activated aluminum-water reaction for direct hydrogen generation at room temperature[J]. Renewable And Sustainable Energy Reviews, 2018, 92: 17-37. |
| 4 | Gai W Z, Liu W H, Deng Z Y, Et al. Reaction of al powder with water for hydrogen generation under ambient condition[J]. International Journal of Hydrogen Energy, 2012, 37: 13132-13140. |
| 5 | Yavor Y, Goroshin S, Bergthorson J M, et al. Comparative reactivity of industrial metal powders with water for hydrogen production[J]. International Journal of Hydrogen Energy, 2015, 40: 1026-1036. |
| 6 | Eom K S, Kwon J Y, Kim M J, et al. Design of al-fe alloys for fast on-board hydrogen production from hydrolysis[J]. Journal of Materials Chemistry, 2011, 21: 13047-13051. |
| 7 | Eom K S, Kim M J, Oh S K, et al. Design of ternary al-sn-fe alloy for fast on-board hydrogen production, and its application to pem fuel cell[J]. International Journal of Hydrogen Energy, 2011, 36: 11825-11831. |
| 8 | Nithiya A, Saffarzadeh A, Shimaoka T. Hydrogen gas generation from metal aluminum-water interaction in municipal solid waste incineration (Mswi) Bottom ash[J]. Waste Management, 2018, 73: 342-350. |
| 9 | Chen X Y, Zhao Z W, Hao M M, et al. Research of hydrogen generation by the reaction of al-based materials with water[J]. Journal of Power Sources, 2013, 222: 188-195. |
| 10 | Kravchenko O V, Semenenko K N, Bulychev B M, et al. Activation of aluminum metal and its reaction with water[J]. Journal of Alloys and Compounds, 2005, 397: 58-62. |
| 11 | Wang H Z, Leung D Y C, Leung M K H, et al. A review on hydrogen production using aluminum and aluminum alloys[J]. Renewable and Sustainable Energy Reviews, 2009, 13: 845-853. |
| 12 | Zou H B, Chen S Z, Zhao Z H, et al. Hydrogen production by hydrolysis of aluminum[J]. Journal of Alloys and Compounds, 2013, 578: 380-384. |
| 13 | Cuomo J J, Woodall J M. Solid state renewable energy supply[P]. US: 4358291. |
| 14 | Ziebarth T, Woodall J M, Kramer R A, et al. Liquid phase-enabled reaction of a1-ga and A1-Ga-In-Sn alloys with water[J]. International Journal of Hydrogen Energy, 2011, 36: 5271-5279. |
| 15 | Wang W, Zhao X M, Chen D M, et al. Insight into the reactivity of Al-Ga-In-Sn alloy with water[J]. International Journal of Hydrogen Energy, 2012, 37: 2187-2194. |
| 16 | He T T, Wang W, Chen W, et al. Influence of in and sn compositions on the reactivity of Al-Ga-In-Sn alloys with water[J]. International Journal of Hydrogen Energy, 2017, 42: 5627-5637. |
| 17 | 贺甜甜. 活性铝合金-水体系产氢性能及机理研究[D]. 北京:中国科学院大学, 2015. |
| He Tian-tian. Hydrogen production performance and mechanism of activated aluminum alloy-water system[D]. Beijing:University of Chinese Academy of Sciences, 2015. | |
| 18 | He T T, Wang W, Chen W, et al. Influence of in and sn compositions on the reactivity of Al-Ga-In-Sn alloys with water[J]. International Journal of Hydrogen Energy, 2017, 42: 5627-5637. |
| 19 | 刘昕, 吴天祥, 赵群丽 等. 基于Kriging代理模型对产不饱和脂肪酸的酒曲微生物混菌比例优化[J]. 酿酒科技, 2016(9): 23-27. |
| Liu Xin, Wu Tian-xiang, Zhao Qun-li, et al. Optimization of mixed ratio of microbial strains to produce unsaturated fatty acids based on kriging model[J]. Liquor-making Science & Technology, 2016(9): 23-27. |
| No related articles found! |
|
||