| 1 |
赵 丹, 常青云, 张 伟,等. 三磷酸腺苷-肿瘤体外药敏试验预测复发性卵巢上皮癌化疗敏感性的价值[J]. 中华肿瘤杂志,2010, 32(11):855-858.
|
| 2 |
张喜梅, 马国芳, 陈玉芬. 卵巢肿瘤细胞体外药物敏感试验临床应用价值[J]. 中国生化药物杂志,2015, 35(4):84-87.
|
| 3 |
崔 颖, 周 丹, 李 叶,等. 88例卵巢上皮性癌行ATP-TCA体外药敏试验结果分析[J]. 中国细胞生物学学报,2014,36(7):989-993.
|
| 4 |
施敏凤. 人卵巢癌肿瘤干细胞样细胞的分离、鉴定及对化疗药物敏感性的研究[D].杭州:浙江大学,2010.
|
| 5 |
HARPSØE N G, ANDERSEN L P, GÖGENUR I,et al.Clinical pharmacokinetics of melatonin: a systematic review[J]. Eur J Clin Pharmacol, 2015, 71(8): 901-909.
|
| 6 |
NUTHALAPATI S, MUNASINGHE W, GIRANDA V,et al. Clinical pharmacokinetics and mass balance of veliparib in combination with temozolomide in subjects with nonhematologic malignancies[J]. Clin Pharmacokinet, 2018, 57(1): 51-58.
|
| 7 |
O’DWYER P J, STEVENSON J P, JOHNSON S W. Clinical pharmacokinetics and administration of established platinum drugs[J].Drugs,2000,59():19-27.
|
| 8 |
OHTA I, GORAI I, MIYAMOTO Y, et al. Cyclophosphamide and 5-fluorouracil act synergistically in ovarian clear cell adenocarcinoma cells[J]. Cancer Lett, 2001, 162(1): 39-48.
|
| 9 |
OHTSU T, SASAKI Y, TAMURA T, et al. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3-hour infusion versus a 24-hour infusion[J]. Clin Cancer Res, 1995, 1(6): 599-606.
|
| 10 |
王 蓉. 基于程序理论的健康教育路径对卵巢癌术后患者生活质量的效果[J]. 湖北医药学院学报, 2019, 38(2): 182-185.
|
| 11 |
刘小珍. 肿瘤细胞原代培养与保存[J]. 中国肿瘤, 2015, 24(4):276-283.
|
| 12 |
YAN X, ZHOU L, WU Z, et al. High throughput scaffold-based 3D micro-tumor array for efficient drug screening and chemosensitivity testing[J]. Biomaterials, 2019, 198: 167-179.
|
| 13 |
DOUBENI C A, DOUBENI A R, MYERS A E. Diagnosis and management of ovarian cancer[J]. Am Fam Physician, 2016, 93(11): 937-944.
|
| 14 |
KARLSEN M A, SANDHU N, HØGDALL C, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass[J]. Gynecol Oncol, 2012, 127(2): 379-383.
|
| 15 |
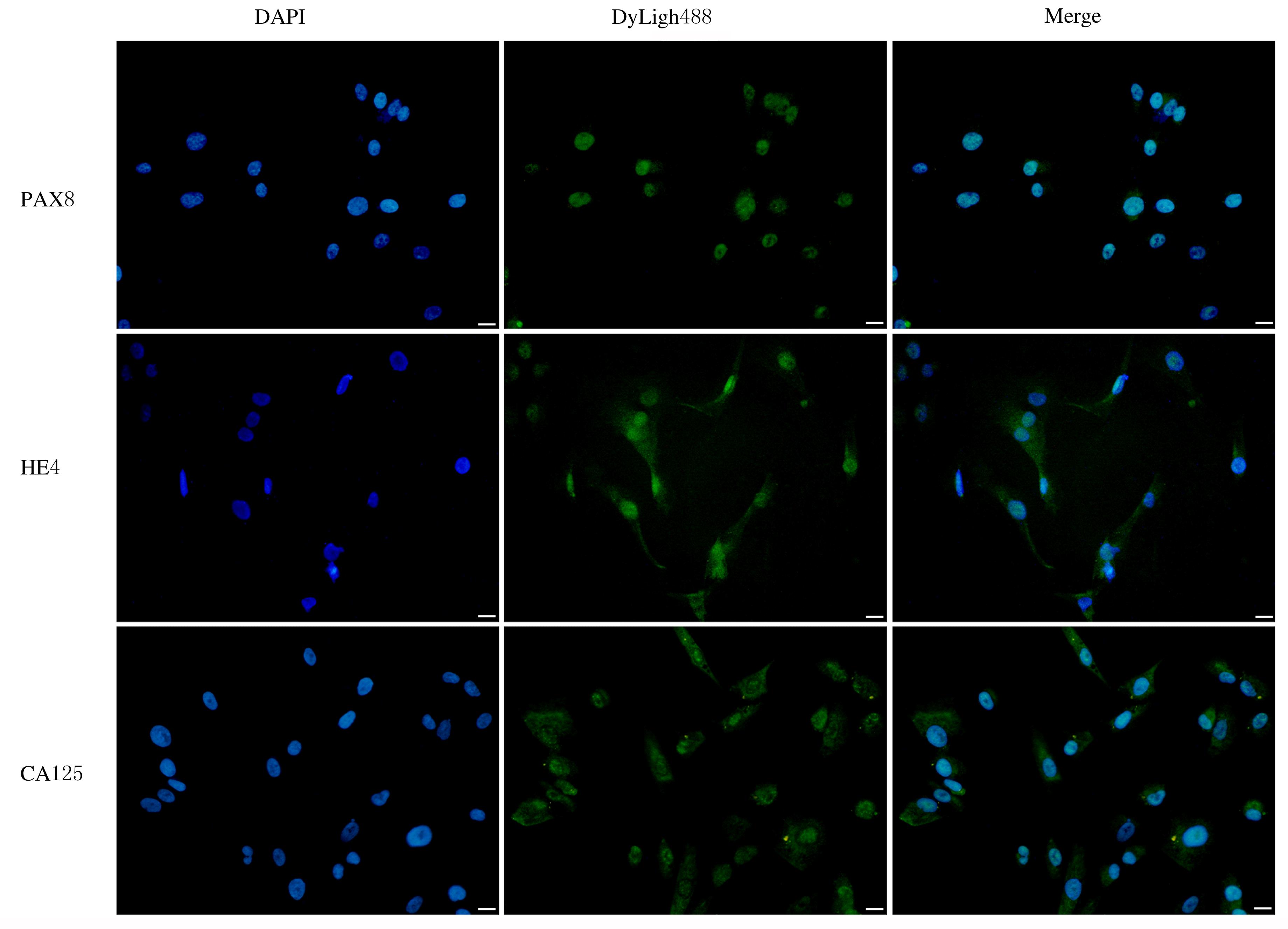
CHAPEL D B, HUSAIN A N, KRAUSZ T, et al. PAX8 expression in a subset of malignant peritoneal mesotheliomas and benign mesothelium has diagnostic implications in the differential diagnosis of ovarian serous carcinoma[J]. Am J Surg Pathol, 2017, 41(12): 1675-1682.
|
| 16 |
CORONA R I, SEO J H, LIN X, et al. Non-coding somatic mutations converge on the PAX8 pathway in ovarian cancer[J]. Nat Commun, 2020, 11(1): 2020.
|
| 17 |
SALMINEN L, GIDWANI K, GRÈNMAN S, et al. HE4 in the evaluation of tumor load and prognostic stratification of high grade serous ovarian carcinoma[J]. Acta Oncol, 2020, 59(12): 1461-1468.
|
| 18 |
WANG Z H, TAO X, YING C M. CPH-I and HE4 are more favorable than CA125 in differentiating borderline ovarian tumors from epithelial ovarian cancer at early stages[J]. Dis Markers, 2019, 2019: 6241743.
|
| 19 |
KHOO S K, HURST T, WEBB M J. Chemosensitivity testing of ovarian cancer: results of a rapid in vitro biochemical assay[J]. Aust N Z J Obstet Gynaecol, 1985, 25(3): 215-220.
|
| 20 |
SCHRAG D, GAREWAL H S, BURSTEIN H J,et al. American society of clinical oncology technology assessment: chemotherapy sensitivity and resistance assays[J]. J Clin Oncol, 2004, 22(17): 3631-3638.
|
| 21 |
苏 丹, 何文静, 刘 屹, 等. 体外药敏实验对卵巢癌个体化化疗的疗效分析[J]. 实用医院临床杂志, 2019, 16(3): 4-7.
|
| 22 |
CREE I A, KURBACHER C M, LAMONT A, et al. A prospective randomized controlled trial of tumour chemosensitivity assay directed chemotherapy versus physician’s choice in patients with recurrent platinum-resistant ovarian cancer[J]. Anti Cancer Drugs, 2007, 18(9): 1093-1101.
|
| 23 |
CHEN L, LIU L P, LI Y H, et al. Melatonin increases human cervical cancer HeLa cells apoptosis induced by cisplatin via inhibition of JNK/Parkin/mitophagy axis[J]. In Vitro Cell Dev Biol Anim, 2018,54(1):1-10.
|
| 24 |
AKBARZADEH M, RAHBARGHAZI R, NABAT E, et al. The impact of different extracellular matrices on melatonin effect in proliferation and stemness properties of ovarian cancer cells[J]. Biomed Pharmacother, 2017, 87: 288-295.
|
| 25 |
LEE J H, YUN C W, HAN Y S, et al. Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regulating cellular prion protein-Oct4 axis[J]. J Pineal Res, 2018, 65(4): e12519.
|
| 26 |
AKBARZADEH M, MOVASSAGHPOUR A A, GHANBARI H, et al. The potential therapeutic effect of melatonin on human ovarian cancer by inhibition of invasion and migration of cancer stem cells[J]. Sci Rep, 2017, 7(1): 17062.
|
 ),孙晓东1,2,3(
),孙晓东1,2,3( )
)
 ),Xiaodong SUN1,2,3(
),Xiaodong SUN1,2,3( )
)