吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (6): 1535-1546.doi: 10.13481/j.1671-587X.20240607
• 基础研究 • 上一篇
浓缩生长因子联合骨髓间充质干细胞膜片的生物学性能及其在骨缺损修复中的作用
- 1.吉林大学口腔医院口腔颌面外一科与口腔整形美容外科,吉林 长春 130021
2.吉林大学口腔医院 吉林省牙发育及颌骨重塑与再生重点实验室,吉林 长春 130021
Biological properties of concentrated growth factor combined with bone marrow mesenchymal stem cell sheet and its effect on bone defect repairment
Jianhong SHI1,2,Yuanye TIAN1,Kai CHEN1,Gao SUN1,Guomin WU1( )
)
- 1.Department of Oral and Maxillofacial Surgery and oral Plastic Surgery,Stomatology Hospital,Jilin University,Changchun 130021,China
2.Jilin Provincal Key Laboratory of Tooth Development and Bone Remodeling and Regeneration,Stomatology Hospital,Jilin University,Changchun 130021,China
摘要:
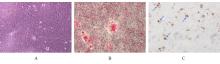
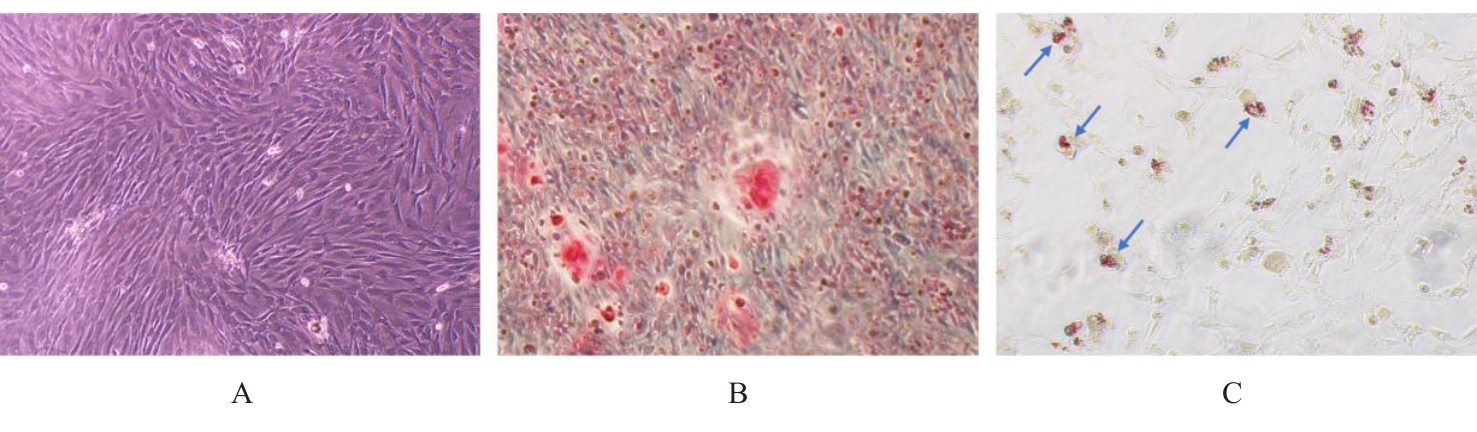
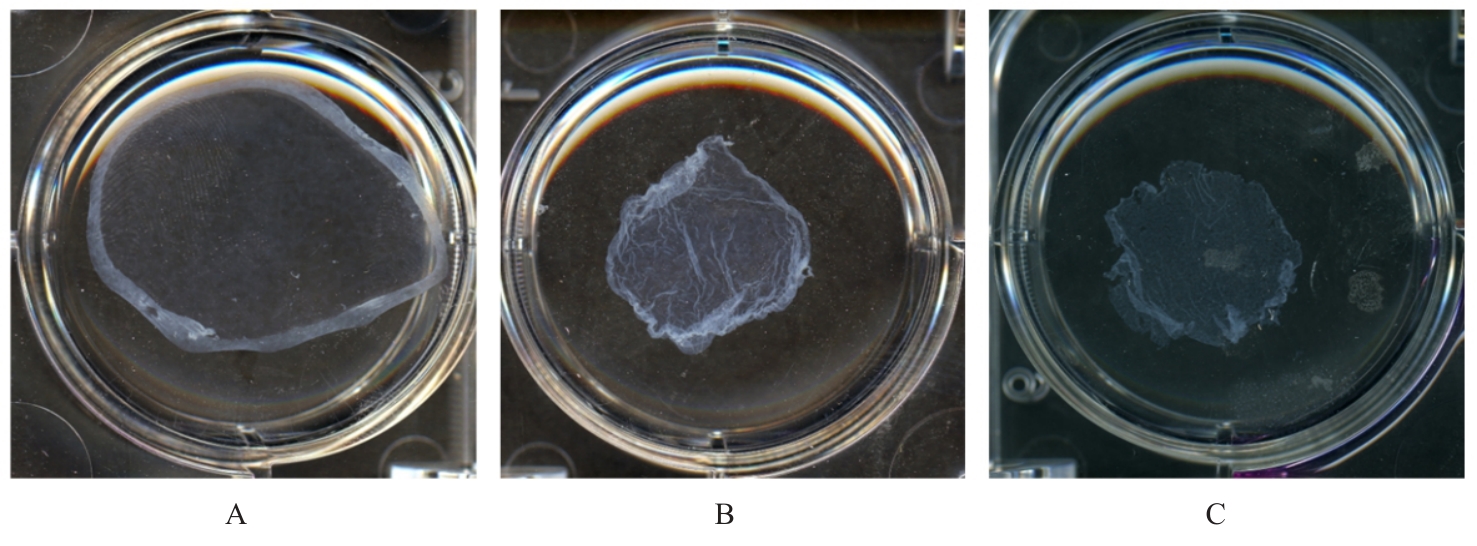
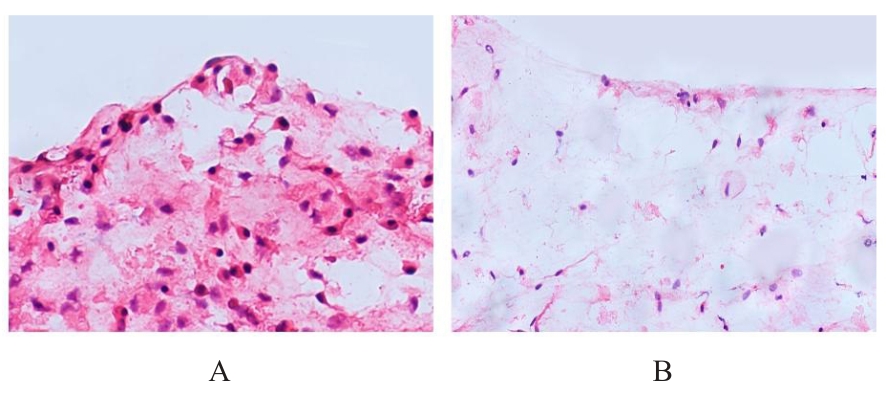
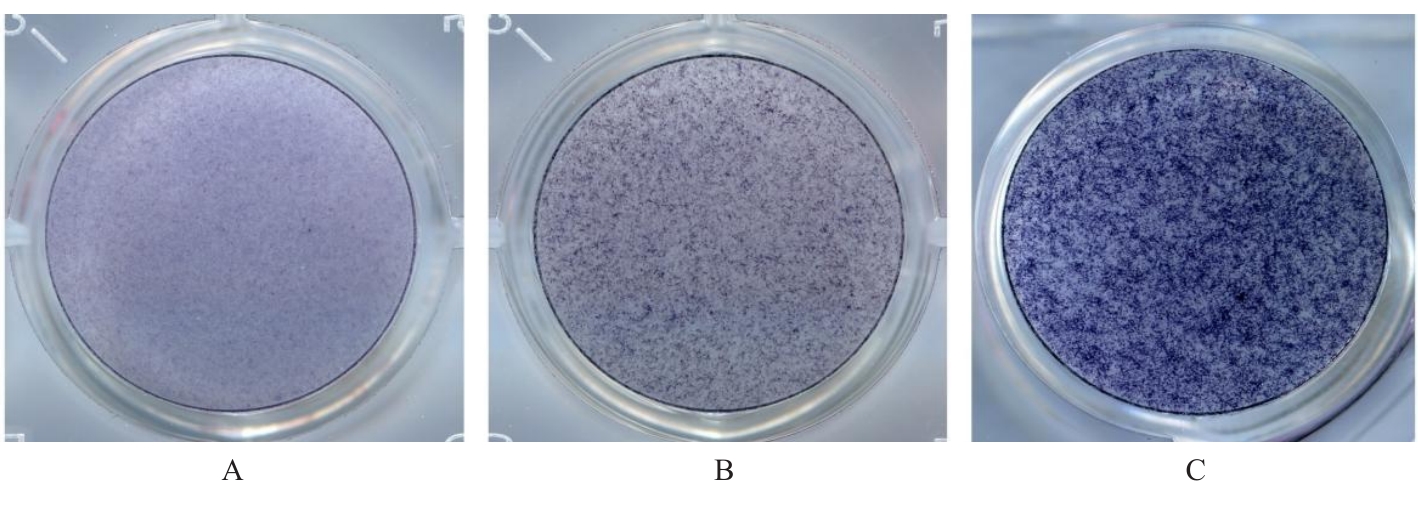
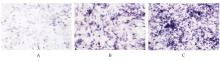
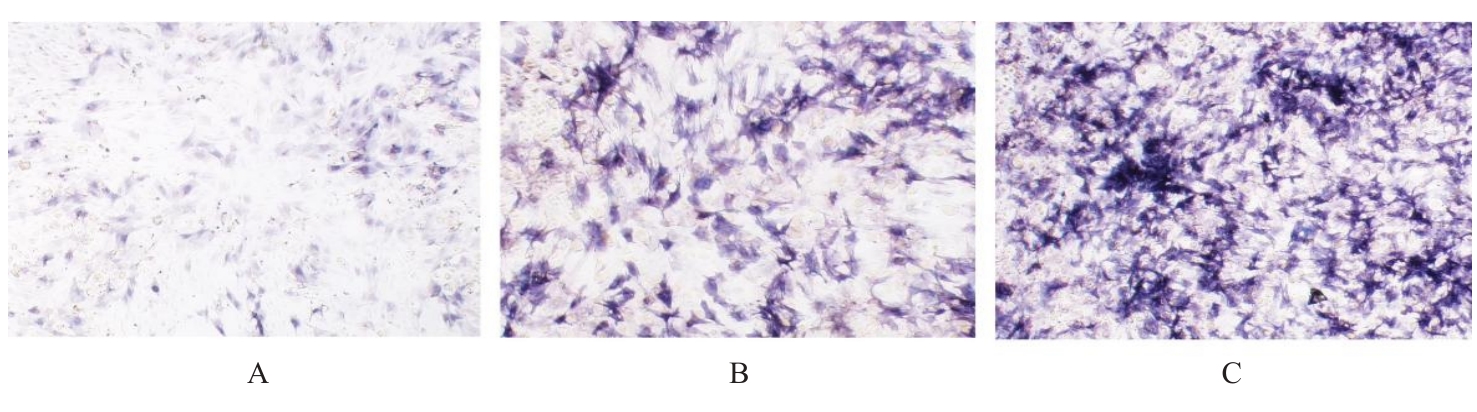
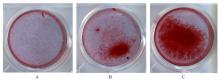
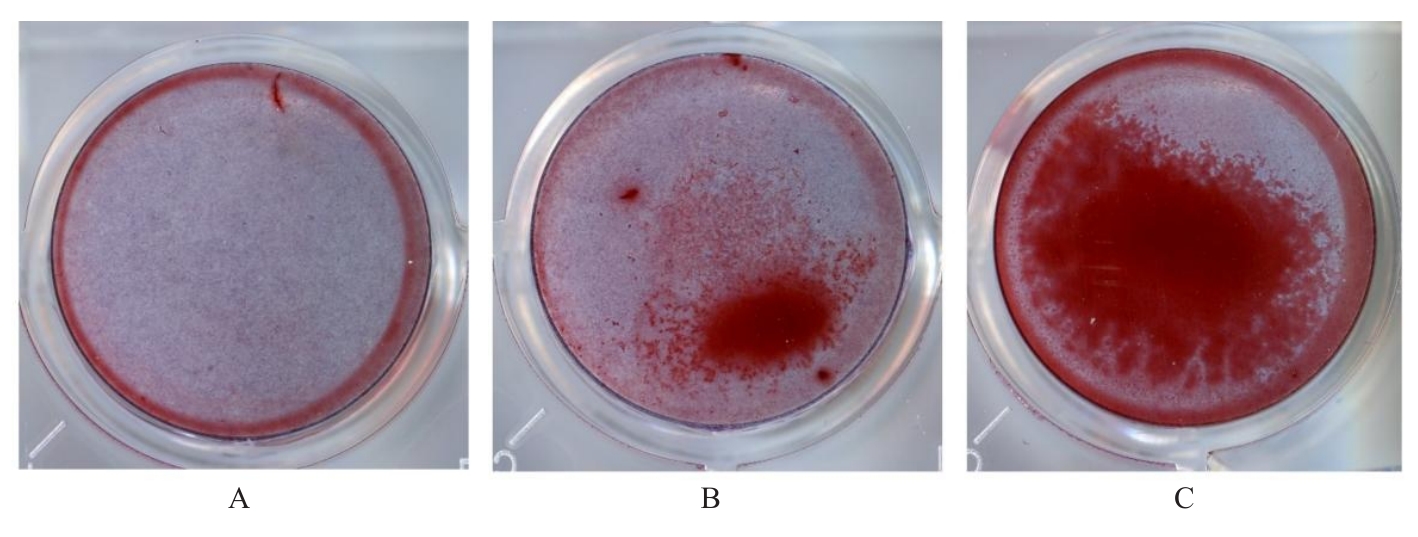
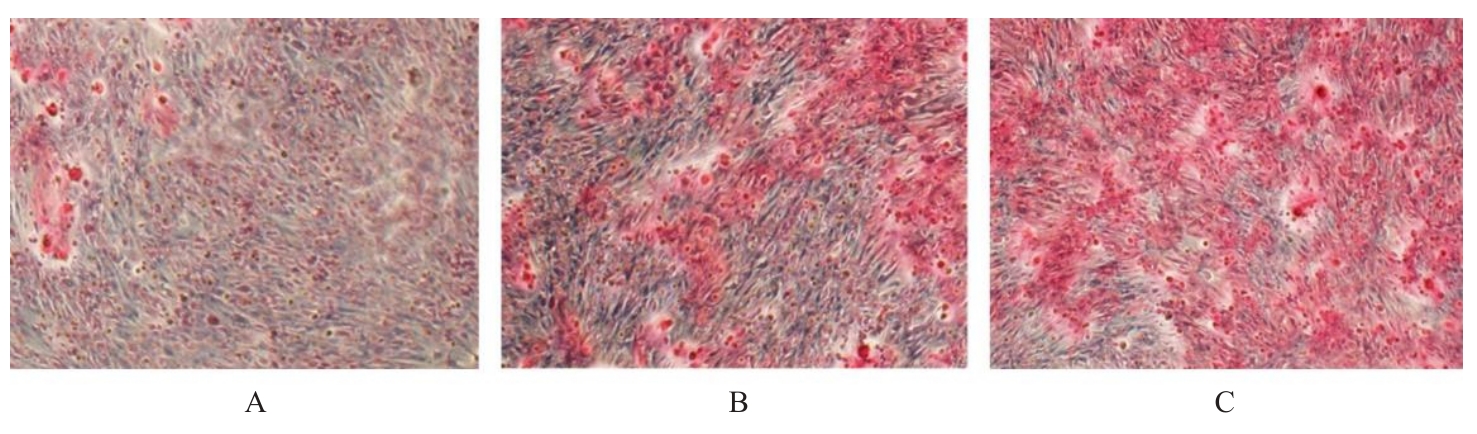
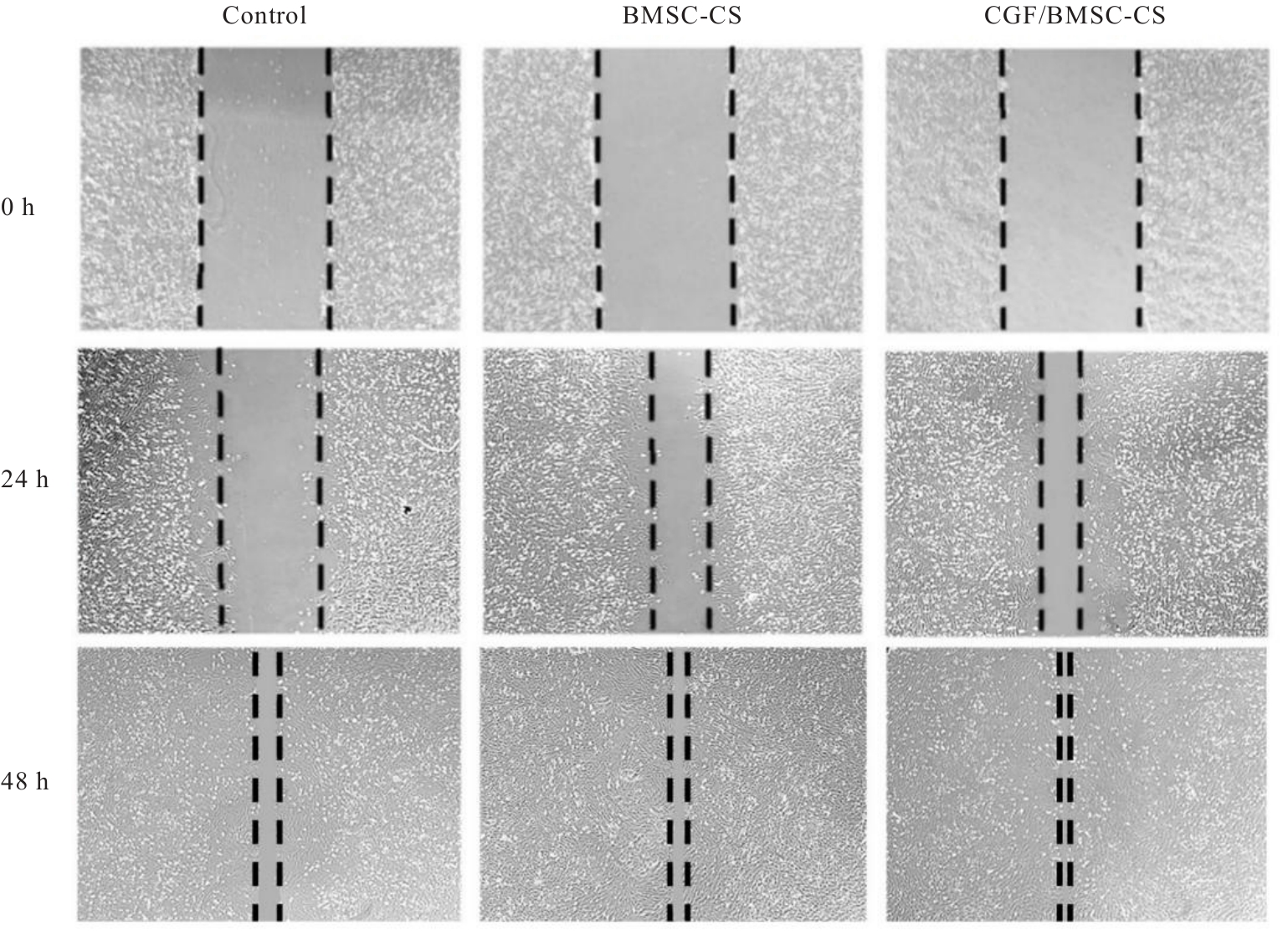
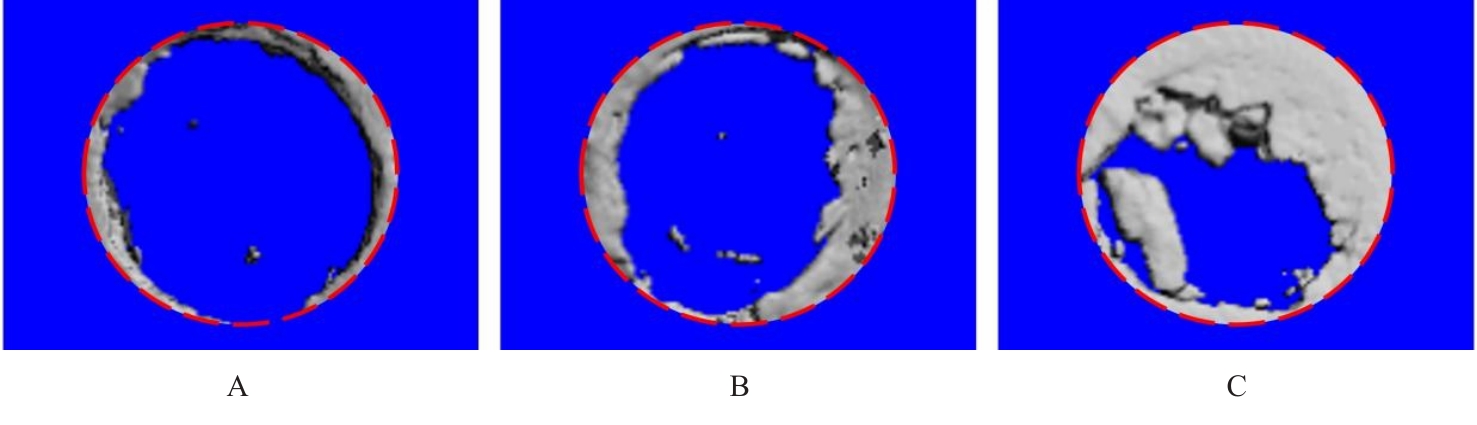
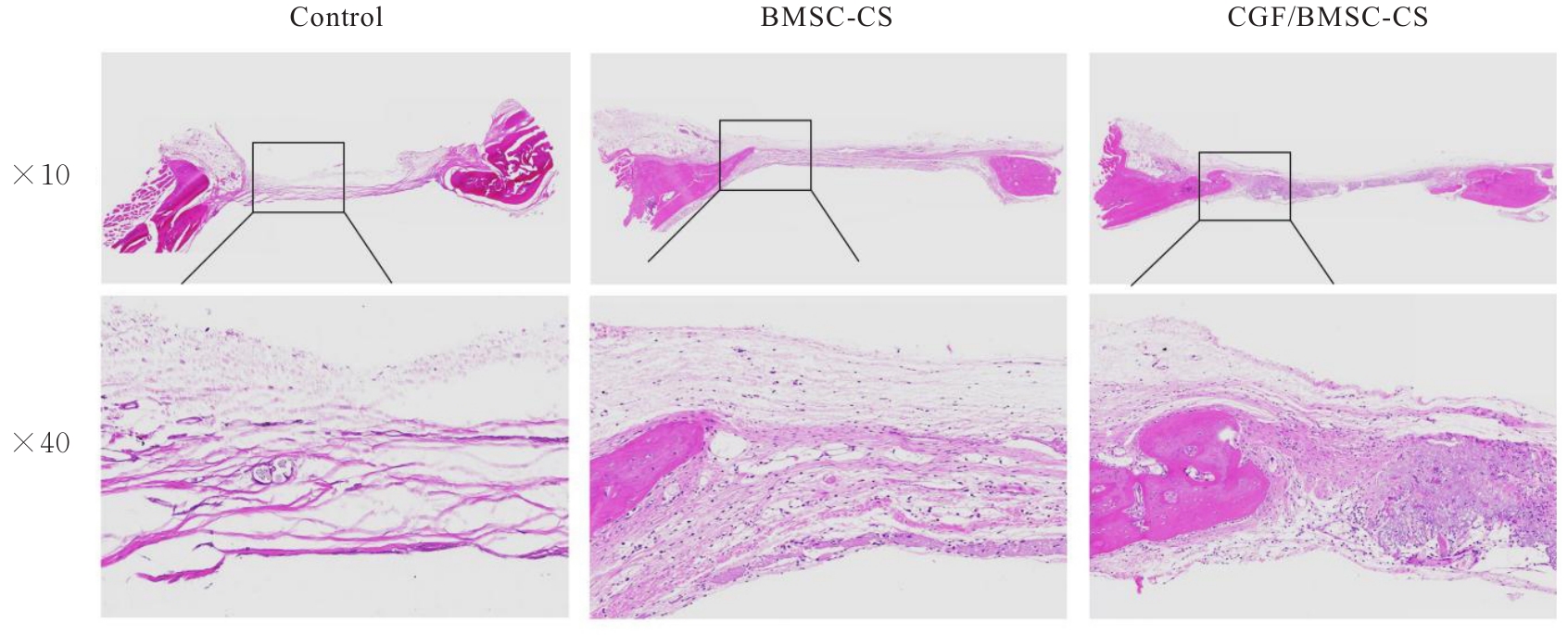
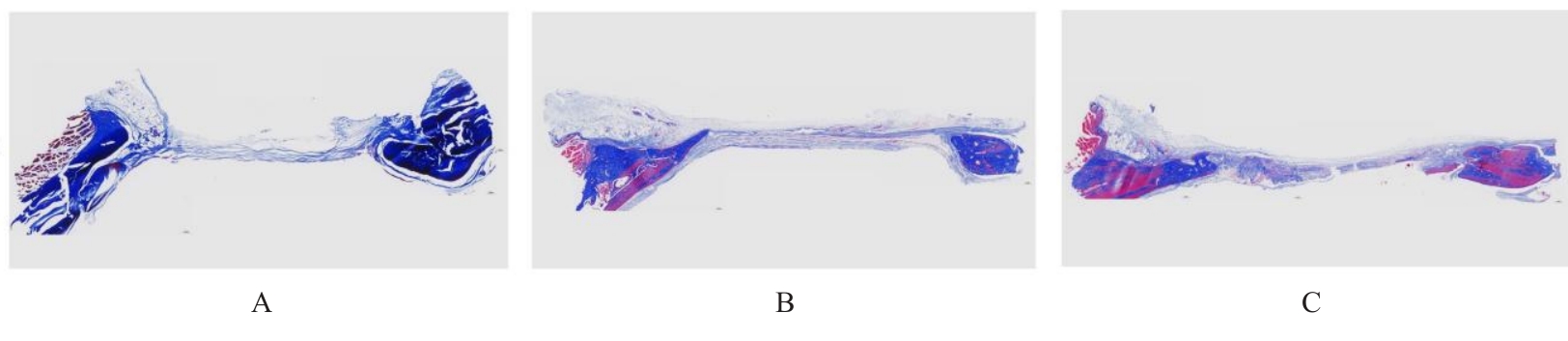
目的 探讨浓缩生长因子(CGF)对骨髓间充质干细胞(BMSCs)膜片性能的影响,并阐明含CGF复合细胞膜片(CS)在骨缺损修复中的作用。 方法 体外实验,选取2只3周龄SD大鼠,分离培养获得BMSCs,茜素红和油红O染色鉴定BMSCs成骨和成脂能力。选取3只3周龄SD大鼠,制备CGF液态提取物(CGFe),将细胞分为对照组、传统CS(BMSC-CS)组和含CGF复合CS(CGF/BMSC-CS)组。HE染色观察2组CS形态表现,茜素红和碱性磷酸酶(ALP)染色检测各组CS体外成骨情况,细胞划痕实验检测各组细胞迁移能力,实时荧光定量PCR(RT-qPCR)法检测各组细胞中ALP、胶原酶Ⅰ型(COL-1)、Runt相关转录因子2(RUNX2)和骨钙蛋白(OCN)mRNA表达水平。体内实验,选取15只SD大鼠随机分为对照组、BMSC-CS组和CGF/BMSC-CS组,显微计算机断层扫描(Micro-CT)检测各组大鼠颅骨缺损处骨形成参数,HE染色和Masson染色观察各组大鼠颅骨缺损组织形态表现。 结果 第3代BMSCs为梭形,排列紧密,呈漩涡团簇状生长;茜素红染色有明显的钙结节生成,油红O染色有红色脂滴形成,证实细胞具有良好的成骨和成脂分化的能力。CS为白色半透明状,边缘轻微卷曲,剥离后的CS卷曲皱缩为不规则状。与BMSC-CS组比较,CGF/BMSC-CS组CS白色更深,透明程度较低,在厚度和延展性方面明显增加,不易破损,有一定黏性和可塑性。HE染色观察,与BMSC-CS组比较,CGF/BMSC-CS组CS细胞数增加,排列密集,细胞外基质(ECM)更丰富,包裹连接细胞形成一个整体的片状结构。茜素红和ALP染色检测,与对照组比较,BMSC-CS组CS的ALP活性和矿化提升值均明显升高(P<0.05);与对照组和BMSC-CS组比较,CGF/BMSC-CS组CS成骨细胞及红色矿化结节数明显增多,染色明显加深,阳性面积增大,ALP活性和矿化提升值均明显升高(P<0.05)。细胞划痕实验检测,培养24 h,与对照组比较,BMSC-CS组和CGF/BMSC-CS组细胞迁移率均明显升高(P<0.05);与BMSC-CS组比较,CGF/BMSC-CS组细胞迁移率明显升高(P<0.01);培养48 h,与对照组比较,CGF/BMSC-CS组细胞迁移率明显升高(P<0.05)。RT-qPCR法检测,与对照组比较,BMSC-CS组细胞中COL-1和OCN mRNA表达水平均明显升高(P<0.01),CGF/BMSC-CS组细胞中ALP、COL-1、OCN和RUNX2 mRNA表达水平均明显升高(P<0.01);与BMSC-CS组比较,CGF/BMSC-CS组细胞中ALP、COL-1和OCN mRNA表达水平均明显升高(P<0.01)。Micro-CT检测,对照组大鼠颅骨缺损区域边界清晰,几乎无新骨生成;BMSC-CS组大鼠颅骨仅在骨缺损边缘有少量新骨形成,缺损中心区域有明显空缺;CGF/BMSC-CS组大鼠颅骨新骨沿骨缺损边缘向中心区域生成,修复大部分骨缺损;与对照组比较,BMSC-CS组大鼠颅骨骨体积分数[骨体积(BV)/组织体积(TV)]和骨小梁间距(Tb.N)均明显升高(P<0.05),CGF/BMSC-CS组大鼠颅骨BV、BV/TV、骨小梁数目(Tb.Th)和Tb.N均明显升高(P<0.05);与BMSC-CS组比较,CGF/BMSC-CS组大鼠颅骨BV、BV/TV、Tb.Th和Tb.N均明显升高(P<0.01)。HE和Masson染色观察,对照组大鼠颅骨缺损组织几乎无新骨生成,仅见大量胶原纤维连接两侧骨断端;BMSC-CS组大鼠颅骨缺损组织仅在骨缺损边缘有少量新骨形成,中央为致密的胶原纤维与缺损边缘的新生骨连接;CGF/BMSC-CS组大鼠颅骨缺损组织除在骨缺损边缘可以看到新生骨组织外,缺损中央亦有骨岛形成,骨岛周围可见骨细胞及大量的胶原纤维。Masson染色观察,细胞质和类骨质呈红色,胶原呈蓝色;CGF/BMSC-CS组大鼠颅骨缺损组织中可见明显的新形成的类骨质,新骨形成量最高。 结论 CGF可以促进BMSCs膜片的成骨分化和ECM的丰富度,含CGF复合CS可以高效修复大鼠颅骨缺损,是一种理想和安全的促骨再生材料。
中图分类号:
- R78