吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (3): 728-738.doi: 10.13481/j.1671-587X.20240317
• 基础研究 • 上一篇
miR-487a通过靶向调控TIA1对胃癌肿瘤相关巨噬细胞M2型极化的抑制作用
曲颜1,戴霖1,王彪1,阮笃激1,钟裕昌1,杨雪峰1,2( )
)
- 1.遵义医科大学第二附属医院胃肠外科,贵州 遵义 563006
2.遵义医科大学附属医院胃肠外科,贵州 遵义 563000
Inhibitory effect of miR-487a on M2-type polarization of gastric cancer tumor-associated macrophages by targeting TIA1
Yan QU1,Lin DAI1,Biao WANG1,Duji RUAN1,Yuchang ZHONG1,Xuefeng YANG1,2( )
)
- 1.Department of Gastrointestinal Surgery,Second Affiliated Hospital,Zunyi Medical University,Zunyi 563006,China
2.Department of Gastrointestinal Surgery,Affiliated Hospital,Zunyi Medical University,Zunyi 563000,China
摘要:
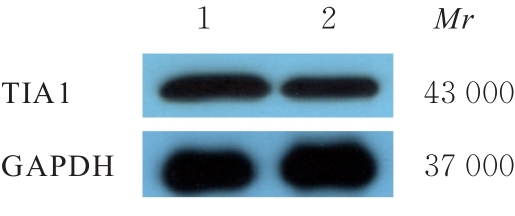
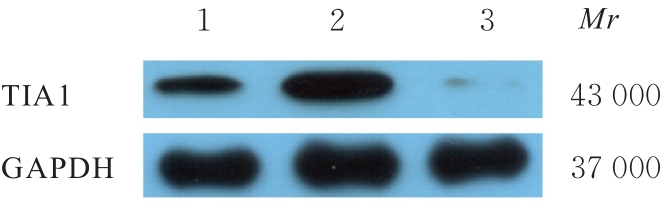
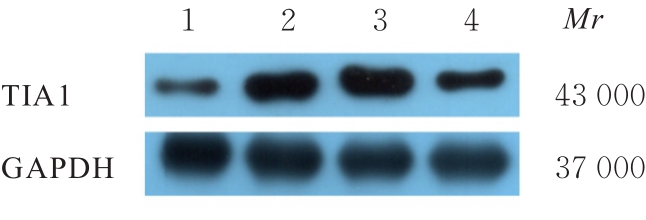
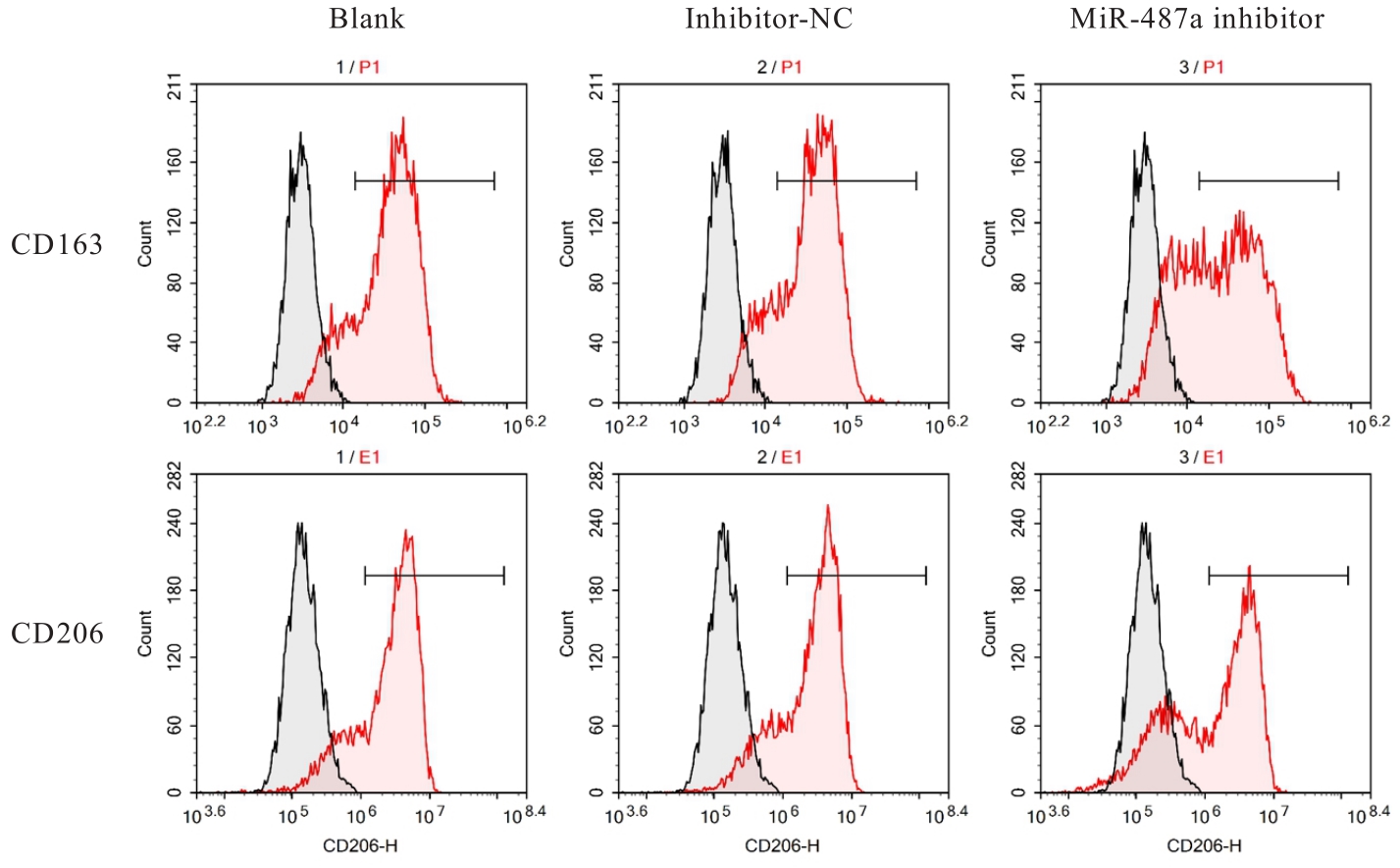
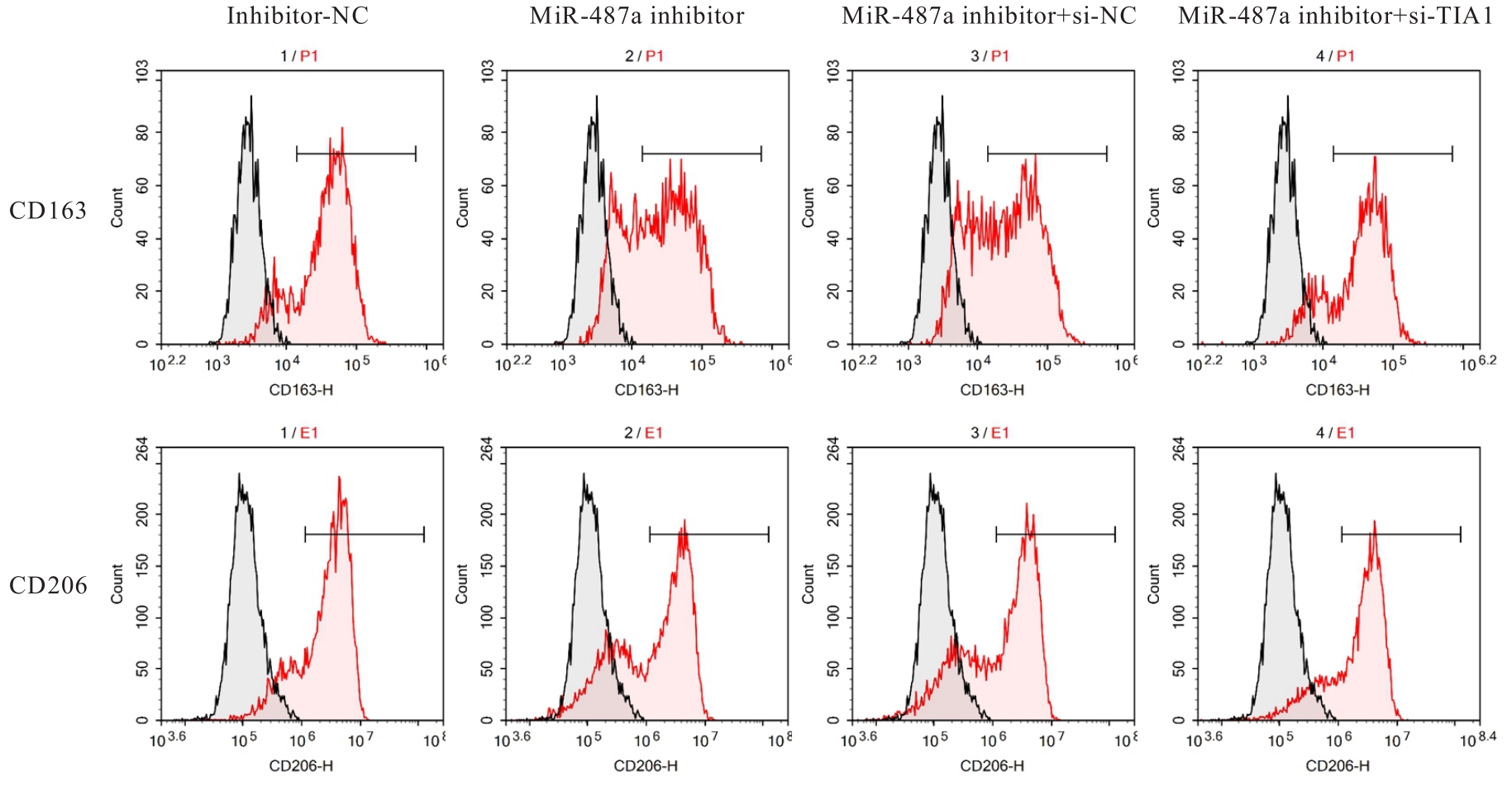
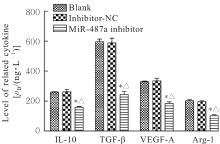
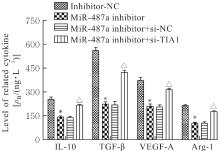
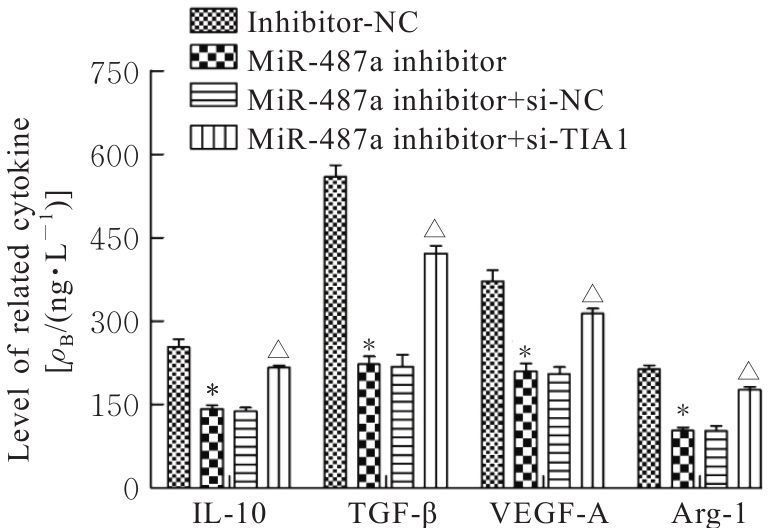
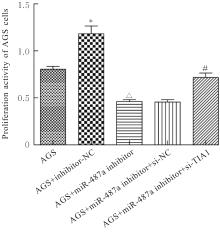
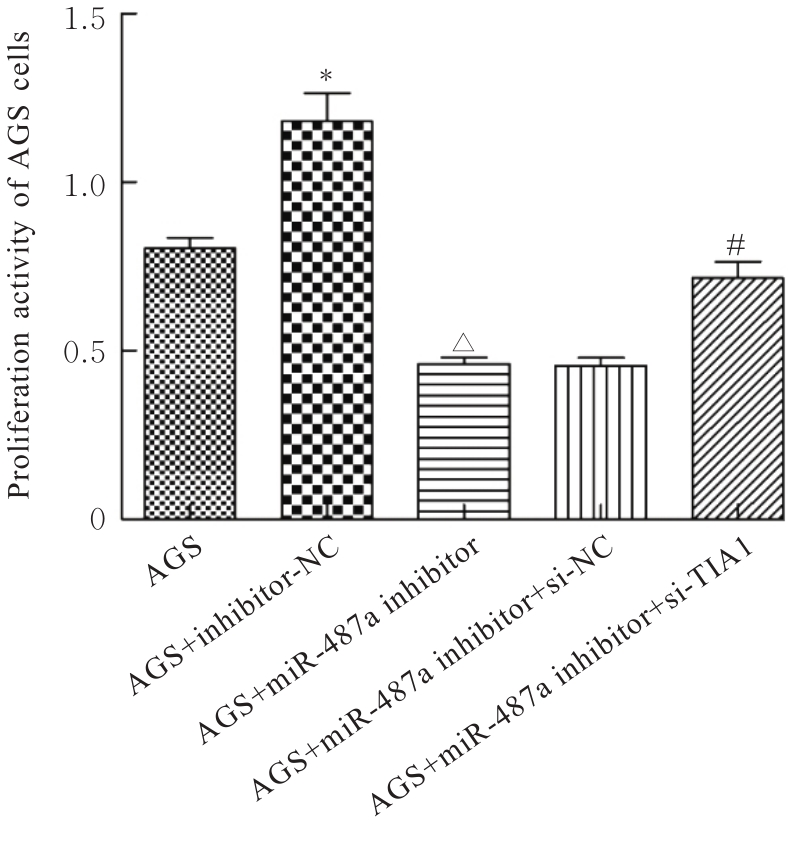
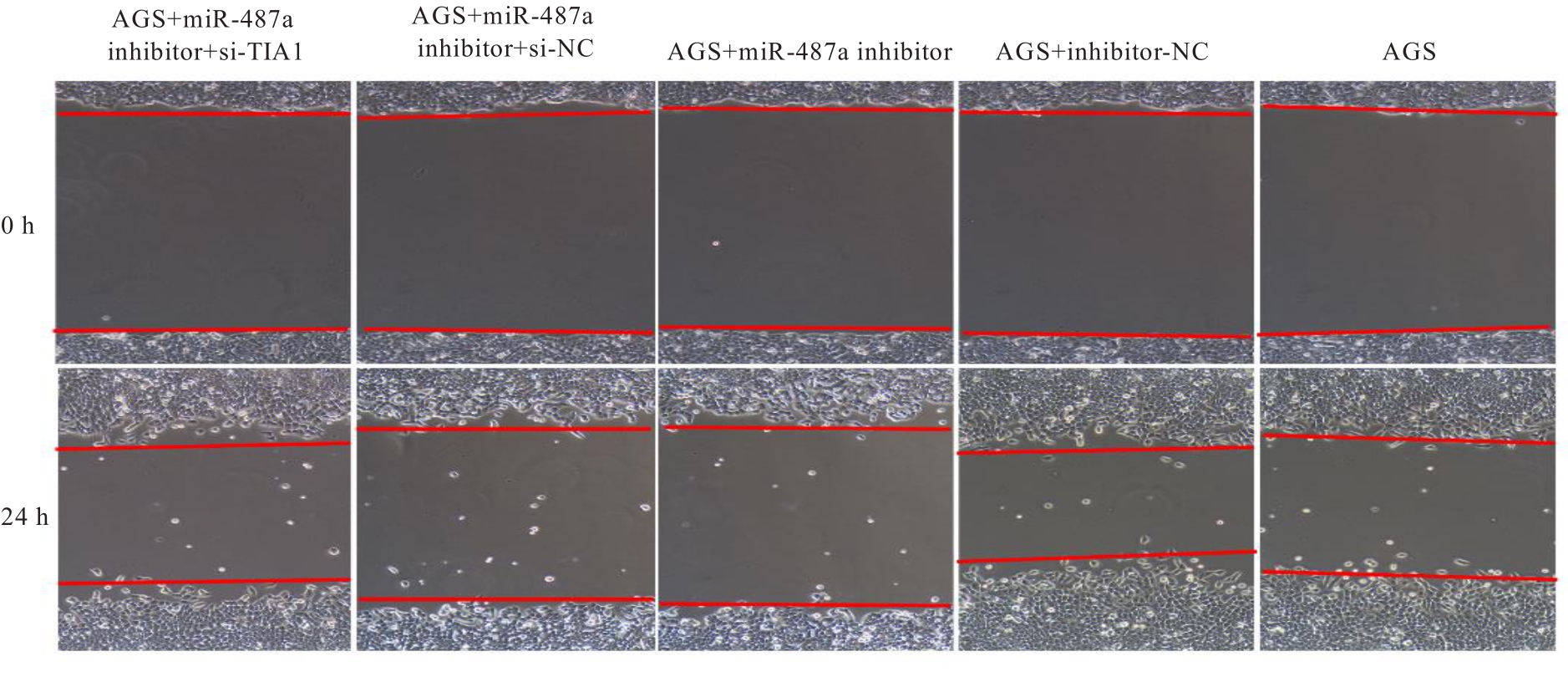
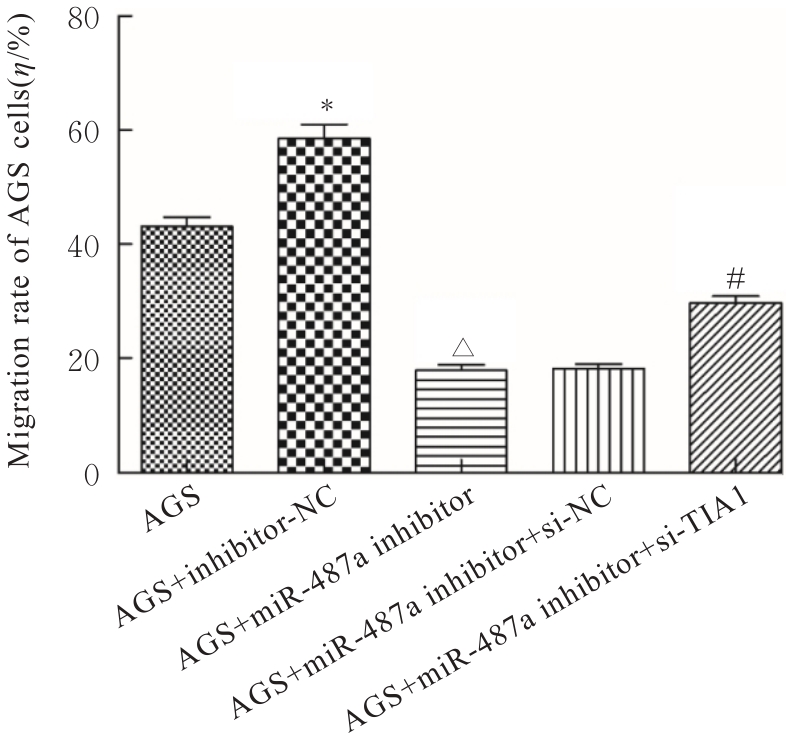
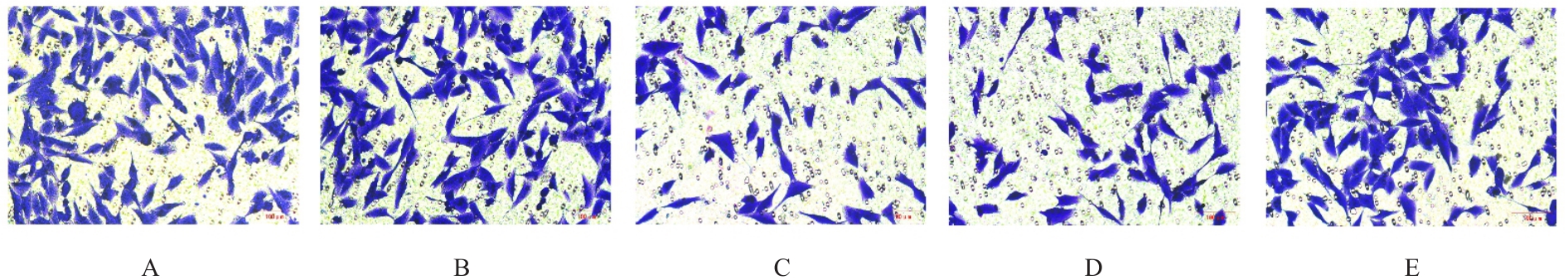
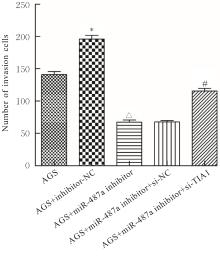
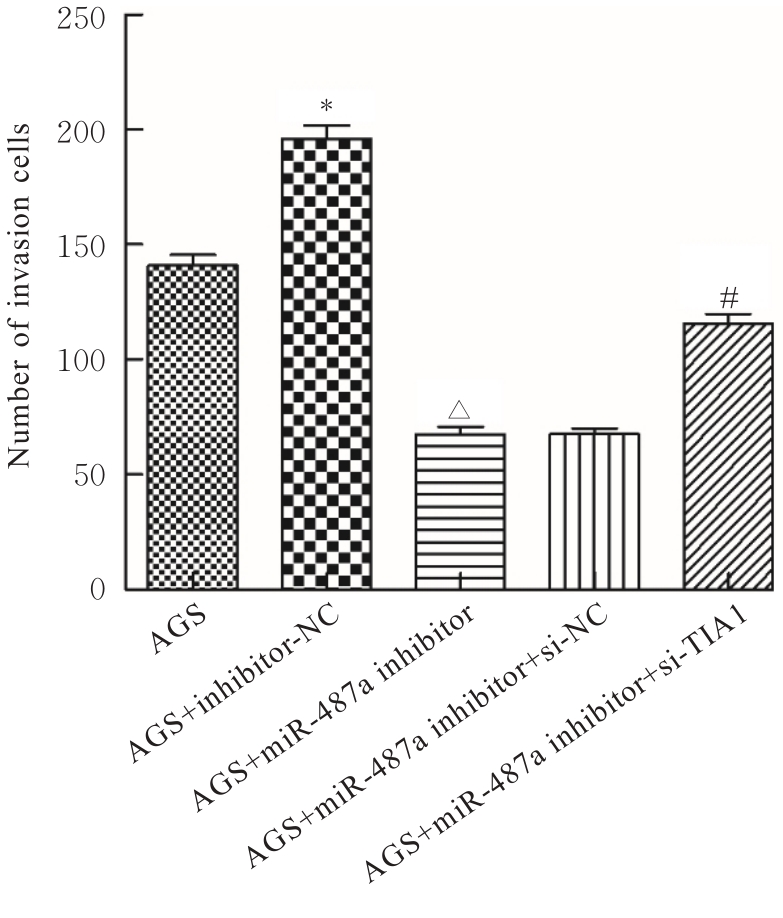
目的 探讨微小RNA(miR)-487a对胃癌肿瘤相关巨噬细胞(TAMs)M2型极化的抑制作用,并阐明其对胃癌AGS细胞增殖、侵袭和迁移的影响。 方法 分离和培养原发性胃癌患者胃癌组织TAMs及癌旁组织来源的正常巨噬细胞(NTMs),体外诱导人单核细胞THP-1分化为TAMs,将分化得到的M0、M1和M2型巨噬细胞经条件培养基(CM)刺激培养24 h,分别获取TAMs、M1-TAMs和M2-TAMs。转染TAMs,分为空白组、inhibitor-NC组、miR-487a inhibitor组、miR-487a inhibitor+si-NC组和miR-487a inhibitor+si-TIA1组,采用实时荧光定量PCR(RT-qPCR)法和Western blotting法验证转染效率。将M2-TAMs与AGS细胞共培养,分为AGS组、AGS+inhibitor-NC组、AGS+miR-487a inhibitor 组、 AGS+miR-487a inhibitor +si-NC 组和 AGS+miR-487a inhibitor+si-TIA1组,RT-qPCR法检测胃癌组织TAMs和癌旁组织NTMs及各组TAMs中miR-487a和T淋巴细胞胞浆内抗原-1(TIA1)mRNA表达水平,Western blotting法检测胃癌组织TAMs和癌旁组织NTMs及各组TAMs中TIA1蛋白表达水平,流式细胞术检测各组TAMs中CD206和CD163水平,酶联免疫吸附试验(ELISA)法检测各组TAMs培养上清中白细胞介素10(IL-10)、转化生长因子β(TGF-β)、血管内皮生长因子A(VEGF-A)和精氨酸酶1(Arg-1)水平,CCK-8法检测各组AGS细胞增殖活性,细胞划痕实验检测各组AGS细胞迁移率,Transwell实验检测各组AGS细胞侵袭细胞数。 结果 RT-qPCR法,与癌旁组织NTMs比较,胃癌组织TAMs中miR-487a表达水平明显升高(P<0.01),TIA1 mRNA表达水平明显降低(P<0.01);与TAMs比较,M1-TAMs中miR-487a表达水平明显降低(P<0.01),TIA1 mRNA表达水平明显升高(P<0.01);M2-TAMs中miR-487a表达水平明显升高(P<0.01),TIA1 mRNA表达水平明显降低(P<0.01);转染后,与空白组和inhibitor-NC组比较,miR-487a inhibitor组细胞中miR-487a表达水平明显降低(P<0.01),提示细胞转染成功。Western blotting法,与癌旁组织NTMs比较,胃癌组织TAMs中TIA1蛋白表达水平明显降低(P<0.01);与TAMs比较,M1-TAMs中TIA1蛋白表达水平明显升高(P<0.01),M2-TAMs中TIA1蛋白表达水平明显降低(P<0.01);共转染后,与inhibitor-NC组比较,miR-487a inhibitor组细胞中TIA1蛋白表达水平明显升高(P<0.01);与miR-487a inhibitor+si-NC组比较,miR-487a inhibitor+si-TIA1组细胞中TIA1蛋白表达水平明显降低(P<0.01)。流式细胞术,与空白组和inhibitor-NC组比较,miR-487a inhibitor组细胞中CD206和CD163水平明显降低(P<0.01);共转染后,与inhibitor-NC组比较,miR-487a inhibitor组细胞中CD206和CD163水平均明显降低(P<0.01);与miR-487a inhibitor+si-NC组比较,miR-487a inhibitor+si-TIA1组细胞中CD206和CD163水平均明显升高(P<0.01)。ELISA法,与空白组和inhibitor-NC组比较,miR-487a inhibitor组TAMs细胞培养上清中IL-10、TGF-β、VEGF-A和Arg-1水平均明显降低(P<0.01);共转染后,与inhibitor-NC组比较,miR-487a inhibitor组TAMs细胞培养上清中IL-10、TGF-β、VEGF-A和Arg-1水平均明显降低(P<0.01);与miR-487a inhibitor+si-NC组比较,miR-487a inhibitor+si-TIA1组TAMs细胞培养上清中IL-10、TGF-β、VEGF-A和Arg-1水平均明显升高(P<0.01)。CCK-8法,与AGS组比较,AGS+inhibitor-NC组细胞增殖活性明显升高(P<0.01);与AGS+inhibitor-NC组比较,AGS+miR-487a inhibitor组细胞增殖活性明显降低(P<0.01);与AGS+miR-487a inhibitor+si-NC组比较,AGS+miR-487a inhibitor +si-TIA1组细胞增殖活性明显升高(P<0.01)。细胞划痕实验,与AGS组比较,AGS+inhibitor-NC组AGS细胞迁移率明显升高(P<0.05);与AGS+inhibitor-NC组比较,AGS+miR-487a inhibitor组AGS细胞迁移率明显降低(P<0.01);与AGS+miR-487a inhibitor+si-NC组比较,AGS+miR-487a inhibitor+si-TIA1组AGS细胞迁移率明显升高(P<0.05)。Transwell实验,与AGS组比较,AGS+inhibitor-NC组AGS细胞侵袭细胞数明显升高(P<0.01);与AGS+inhibitor-NC组比较,AGS+miR-487a inhibitor组AGS细胞侵袭细胞数明显降低(P<0.01);与AGS+miR-487a inhibitor+si-NC组比较,AGS+miR-487a inhibitor+si-TIA1组AGS细胞侵袭细胞数明显升高(P<0.01)。 结论 沉默miR-487a表达可通过靶向上调TIA1抑制胃癌肿瘤相关巨噬细胞M2型极化,并抑制胃癌细胞增殖、迁移和侵袭。
中图分类号:
- R735.2