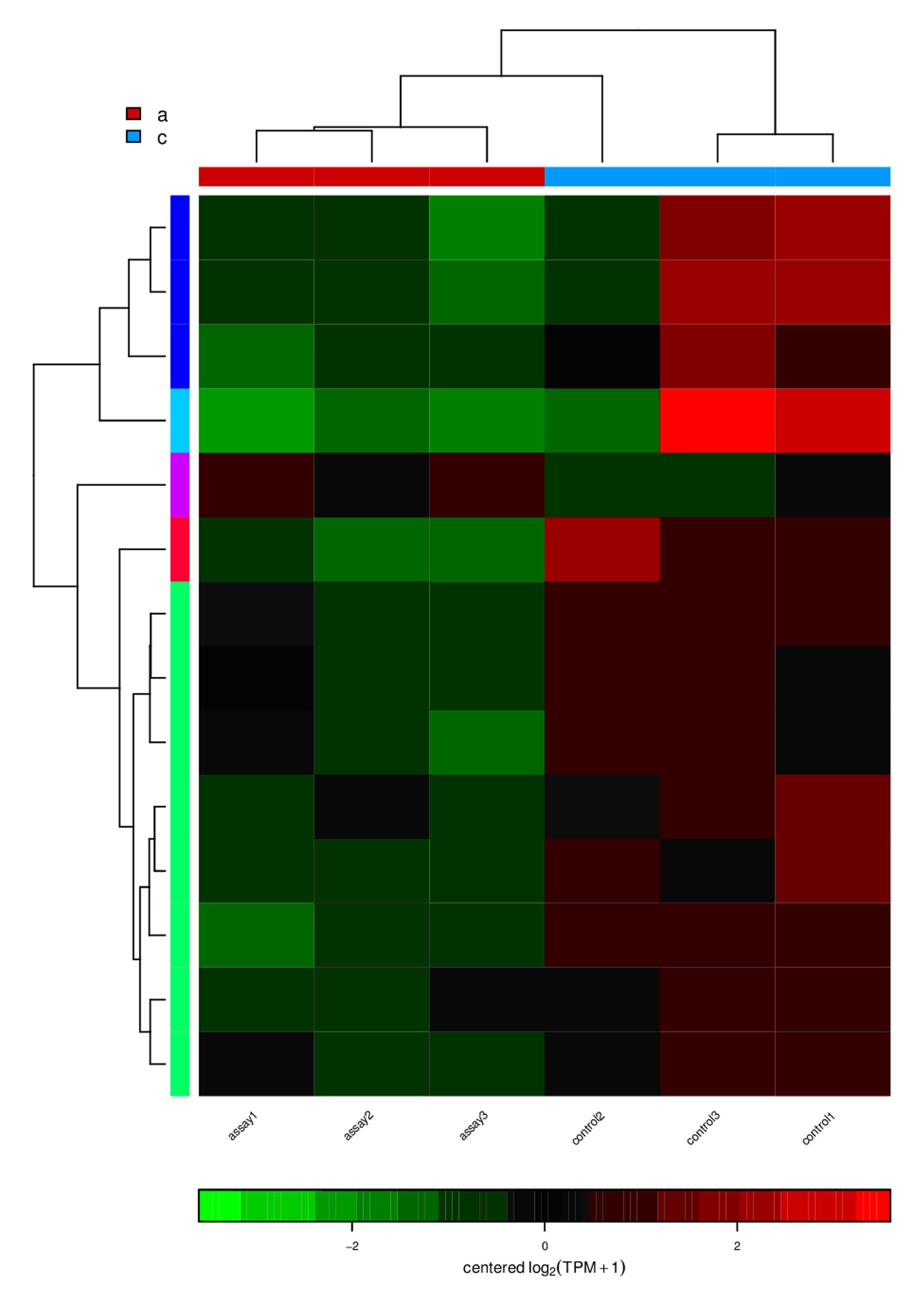
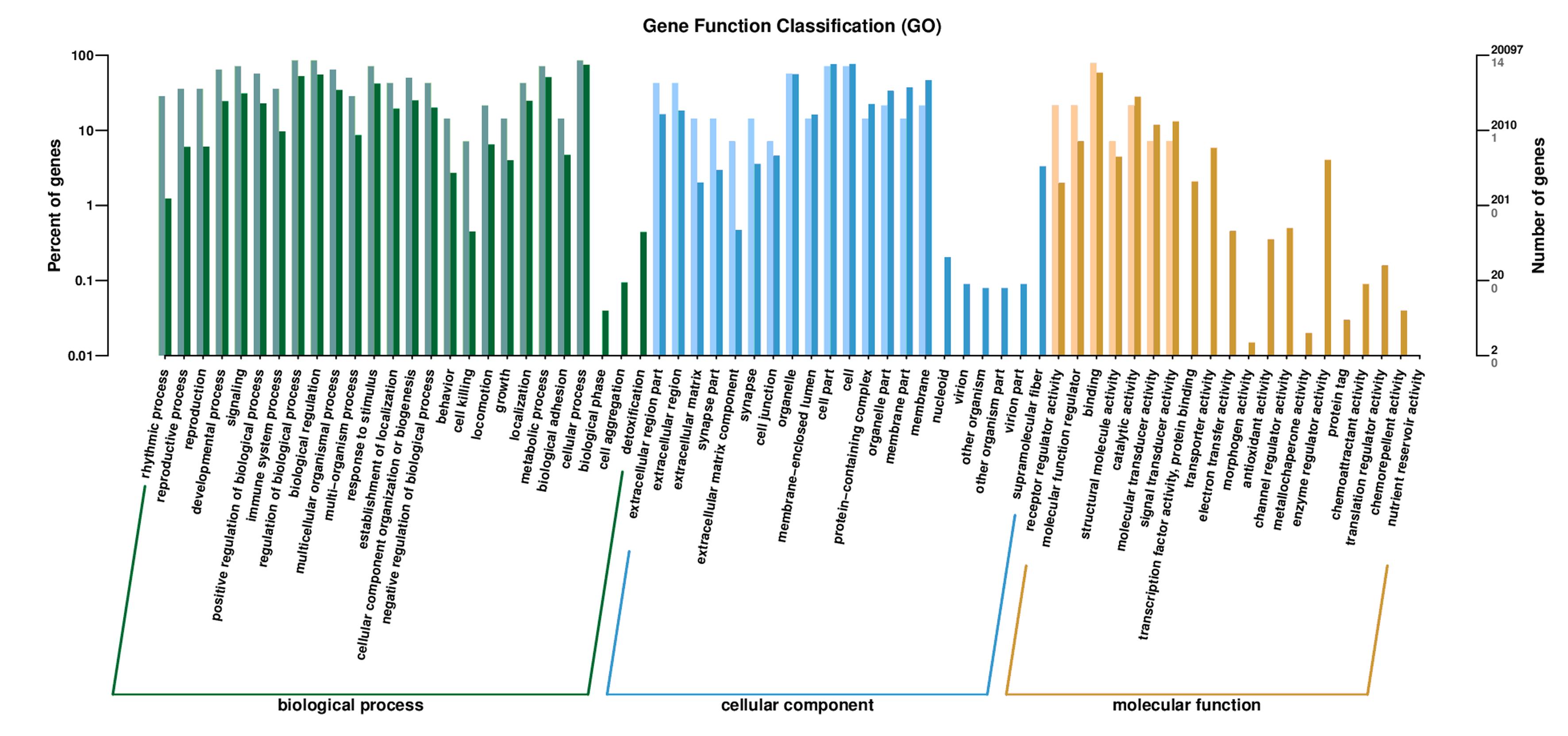
| 1 |
SIFAKIS S, ANDROUTSOPOULOS V P, TSATSAKIS A M, et al. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems[J]. Environ Toxicol Pharmacol, 2017, 51: 56-70.
|
| 2 |
KARWACKA A, ZAMKOWSKA D, RADWAN M, et al. Exposure to modern, widespread environmental endocrine disrupting chemicals and their effect on the reproductive potential of women: an overview of current epidemiological evidence[J]. Hum Fertil(Camb),2019, 22(1): 2-25.
|
| 3 |
HEINDEL J J, BLUMBERG B, CAVE M, et al. Metabolism disrupting chemicals and metabolic disorders[J]. Reproductive Toxicol, 2017, 68: 3-33.
|
| 4 |
SHAFEI A, RAMZY M M, HEGAZY A I, et al. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer[J]. Gene, 2018, 647: 235-243.
|
| 5 |
GEENS T, AERTS D, BERTHOT C, et al. A review of dietary and non-dietary exposure to bisphenol-A[J]. Food Chem Toxicol, 2012, 50(10): 3725-3740.
|
| 6 |
ROCHESTER J R. Bisphenol A and human health: a review of the literature[J]. Reproductive Toxicol, 2013, 42: 132-155.
|
| 7 |
KONIECZNA A, RUTKOWSKA A, RACHOŃ D. Health risk of exposure to Bisphenol A (BPA)[J]. Roczniki Panstwowego Zakl Higieny, 2015,66(1): 5-11.
|
| 8 |
BLOOM M S, KIM D, SVOM SAAL F, et al. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization[J]. Fertil Steril, 2011, 96(3): 672-677.e2.
|
| 9 |
BLOOM M S, MOK-LIN E, FUJIMOTO V Y. Bisphenol A and ovarian steroidogenesis[J]. Fertil Steril, 2016, 106(4): 857-863.
|
| 10 |
PIVONELLO C, MUSCOGIURI G, NARDONE A, et al. Bisphenol A: an emerging threat to female fertility[J]. Reprod Biol Endocrinol, 2020, 18(1): 22.
|
| 11 |
JUNG M K, CHOI H S, SUH J, et al. The analysis of endocrine disruptors in patients with central precocious puberty[J]. BMC Pediatr, 2019, 19(1): 323.
|
| 12 |
ADEWALE H B, JEFFERSON W N, NEWBOLD R R,et al. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons[J].Biol Reprod,2009,81(4): 690-699.
|
| 13 |
RODRÍGUEZ H A, SANTAMBROSIO N, SANTAMARÍA C G, et al. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary[J].Reproductive Toxicol,2010,30(4):550-557.
|
| 14 |
HEINDEL J J. The developmental basis of disease: Update on environmental exposures and animal models[J].Basic Clin Pharmacol Toxicol,2019,125(): 5-13.
|
| 15 |
ZAID S S M, OTHMAN S, KASSIM N M. Protective role of Ficus deltoidea against BPA-induced impairments of the follicular development, estrous cycle, gonadotropin and sex steroid hormones level of prepubertal rats[J]. J Ovarian Res, 2018, 11(1): 99.
|
| 16 |
LI Y, ZHANG W, LIU J, et al. Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression[J]. Reprod Toxicol, 2014, 44: 33-40.
|
| 17 |
CHIANESE R, TROISI J, RICHARDS S, et al. Bisphenol A in reproduction: epigenetic effects[J]. Curr Med Chem, 2018, 25(6): 748-770.
|
| 18 |
WANG Y, SHUANG L, YUJIE S, et al. Activin A overexpression promotes rat follicular development through SCF-kit-mediated cell signals[J]. Gynecol Endocrinol, 2020, 36(12): 1070-1073.
|
| 19 |
LIU Q, LI Y, FENG Y, et al. Single-cell analysis of differences in transcriptomic profiles of oocytes and cumulus cells at GⅤ, MⅠ, MⅡ stages from PCOS patients[J]. Sci Rep, 2016, 6: 39638.
|
| 20 |
SONG J, DIAO F Y, MA X, et al. Androgen upregulates NR4A1 via the TFAP2A and ETS signaling networks[J]. Int J Biochem Cell Biol, 2019, 113: 1-7.
|
| 21 |
QI L, GUO N, WEI Q, et al. The involvement of NR4A1 and NR4A2 in the regulation of the luteal function in rats[J]. Acta Histochem, 2018, 120(8): 713-719.
|
| 22 |
GUZMÁN-ORNELAS M O, PETRI M H, VÁZQUEZ-DEL MERCADO M, et al. CCL2 serum levels and adiposity are associated with the polymorphic phenotypes -2518A on CCL2 and 64ILE on CCR2 in a Mexican population with insulin resistance[J]. J Diabetes Res, 2016, 2016: 1-11.
|
| 23 |
HU M, ZHANG Y, GUO X, et al. Hyperandrogenism and insulin resistance induce gravid uterine defects in association with mitochondrial dysfunction and aberrant reactive oxygen species production[J]. Am J Physiol Endocrinol Metab, 2019, 316(5): E794-E809.
|
| 24 |
CHERMUŁA B, BRĄZERT M, IŻYCKI D, et al. New gene markers of angiogenesis and blood vessels development in porcine ovarian granulosa cells during short-term primary culture in vitro [J]. Biomed Res Int, 2019, 2019: 6545210.
|
| 25 |
ALMARIO R U, KARAKAS S E. Roles of circulating WNT-signaling proteins and WNT-inhibitors in human adiposity, insulin resistance, insulin secretion, and inflammation[J].Horm Metab Res,2015,47(2): 152-157.
|
| 26 |
HAN P, GUERRERO-NETRO H, ESTIENNE A, et al. Regulation and action of early growth response 1 in bovine granulosa cells[J]. Reproduction, 2017, 154(4): 547-557.
|
| 27 |
JIN H, WON M, SHIN E, et al. EGR2 is a gonadotropin-induced survival factor that controls the expression of IER3 in ovarian granulosa cells[J]. Biochem Biophys Res Commun, 2017, 482(4): 877-882.
|
| 28 |
SUN B, MA Y, WANG F, et al. miR-644-5p carried by bone mesenchymal stem cell-derived exosomes targets regulation of p53 to inhibit ovarian granulosa cell apoptosis[J]. Stem Cell Res Ther, 2019, 10(1): 360.
|
| 29 |
ZHANG N, LIU X, ZHUANG L, et al. Berberine decreases insulin resistance in a PCOS rats by improving GLUT4: Dual regulation of the PI3K/AKT and MAPK pathways[J]. Regul Toxicol Pharmacol, 2020, 110: 104544.
|
 )
)