吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (6): 1597-1605.doi: 10.13481/j.1671-587X.20240613
• 基础研究 • 上一篇
罗伊氏乳杆菌对轮状病毒体内外复制的抑制作用及其对免疫因子表达的影响
李小凤1,程美慧2,刘洋1,刘长城1,贾雪娇1,刘梦琦1,赵微1( )
)
- 1.锦州医科大学基础医学院病原生物学实验室,辽宁 锦州 121000
2.湖北省黄石市中心医院检验科,湖北 黄石 435000
Inhibitory effect of Lactobacillus reuteri on rotavirus replication in vivo and in vitro and its effect on expression of immune factors
Xiaofeng LI1,Meihui Cheng2,Yang LIU1,Changcheng LIU1,Xuejiao JIA1,Mengqi LIU1,Wei ZHAO1( )
)
- 1.Laboratory of Pathogenic Biology,School of Basic Medical Sciences,Jinzhou Medical University,Jinzhou 121000,China
2.Department of Laboratory,Central Hospital,Huangshi City,Hubei Province,Huangshi 435000,China
摘要:
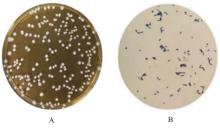
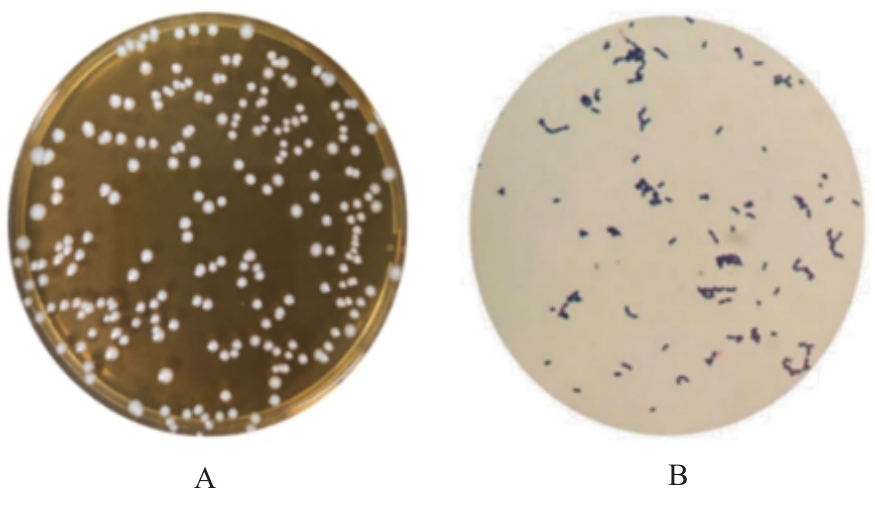
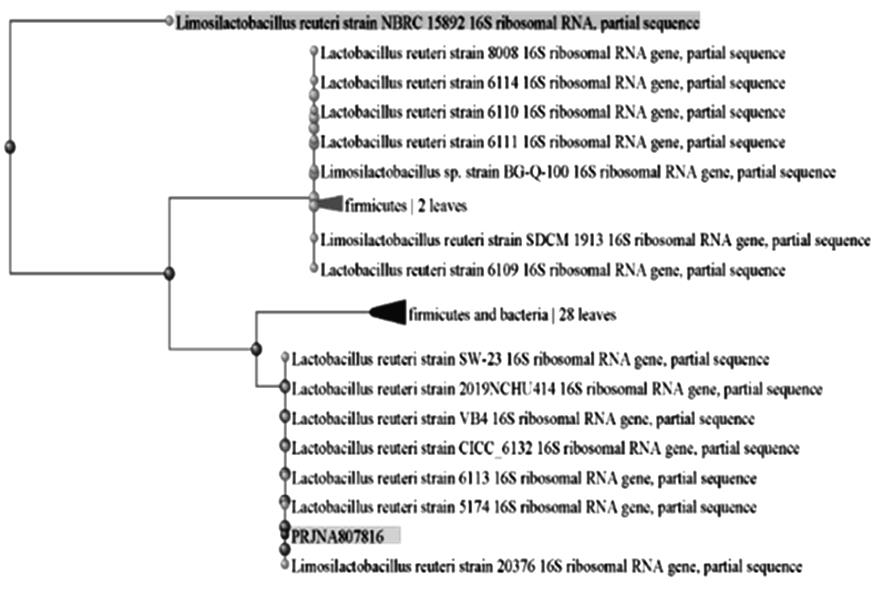
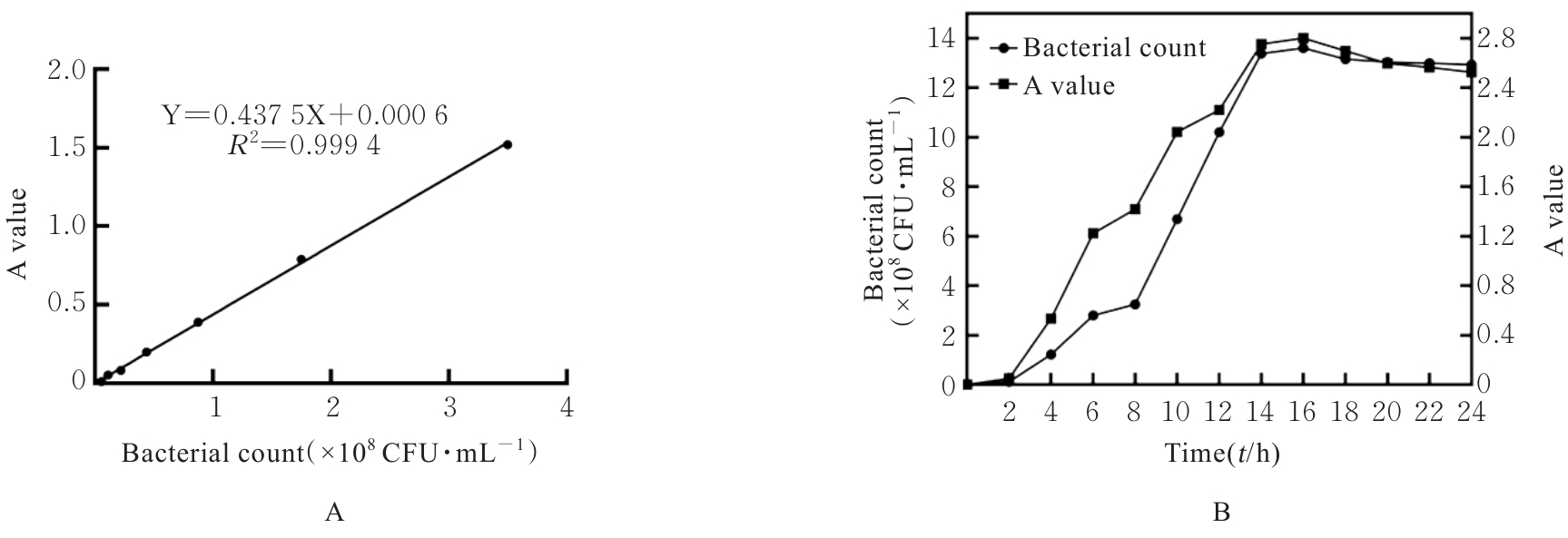
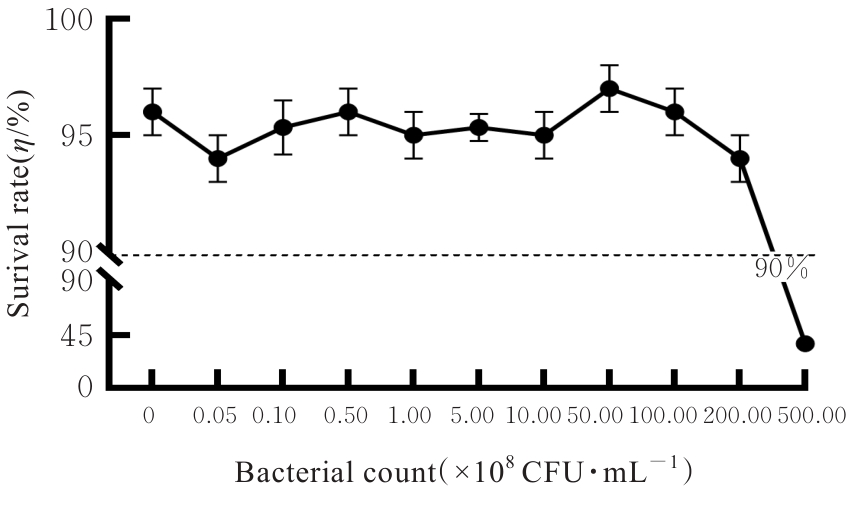
目的 探讨罗伊氏乳杆菌对轮状病毒(RV)SA11株体内外复制的抑制作用,并阐明其对相关免疫因子表达的影响。 方法 体外实验,培养并鉴定罗伊氏乳杆菌,绘制罗伊氏乳杆菌标准曲线和生长曲线,筛选罗伊氏乳杆菌培养最佳时间和最适浓度。采用5×108、10×108、50×108、100×108、200×108和500×108 CFU·mL-1罗伊氏乳杆菌感染细胞,台盼蓝染色法检测Caco-2细胞存活率。将不同浓度罗伊氏乳杆菌与RV体外共孵育并作用于Caco-2细胞,Caco-2细胞分为阴性对照组(NC组)、阳性对照组(PC组)和107、108、109及1010 CFU·mL-1 罗伊氏乳杆菌组,免疫荧光灶法检测罗伊氏乳杆菌作用后Caco-2细胞中病毒滴度,实时荧光定量PCR(RT-qPCR)法检测不同浓度罗伊氏乳杆菌作用后Caco-2细胞中RV VP6基因拷贝数。体内实验,将25窝SPF级乳鼠分为对照组、RV组(感染SA11毒株)、Ab-NC组(抗生素处理耗竭肠道菌群)、Ab-RV组(耗竭肠道菌群后感染SA11毒株)和Ab-Lac-RV组(耗竭肠道菌群,并感染罗伊氏乳杆菌后感染SA11毒株)。收取各组乳鼠灌胃第2、4、6、8和10天的粪便样本和灌胃第4天结肠组织样本,RT-qPCR法检测各组乳鼠粪便中RV VP6基因拷贝数和结肠组织中白细胞介素(IL)-1β、IL-8、IL-10、γ干扰素(IFN-γ)和肿瘤坏死因子(TNF)-α mRNA表达水平。 结果 罗伊氏乳杆菌生长良好,形态圆润,呈圆形、光滑和乳白色的凸起样菌落,且边缘较整齐;革兰染色后菌体呈紫色、不规则和方形杆状;经16SrDNA测序后序列同源性为99%,提示罗伊氏乳杆菌活化成功;罗伊氏乳杆菌活菌数与吸光度(A)值呈线性关系,回归分析标准曲线为Y=0.437 5X+0.000 6,R2=0.999 4。培养0~2 h,细菌处于对数生长期,细菌生长迟缓;培养2~14 h,细菌快速生长,并于培养14~16 h时细菌生长趋于稳定,培养16 h时达到细菌生长速率顶峰,随后进入衰亡期。5×108、10×108、50×108、100×108和200×108 CFU·mL-1罗氏乳杆菌感染后,Caco-2细胞存活率均>90%,因此选用上述浓度罗氏乳杆菌感染细胞。与PC组比较,5×108、10×108、50×108、100×108和200×108 CFU·mL-1罗氏乳杆菌组Caco-2细胞中RV VP6基因拷贝数均明显降低 (P<0.01)。与PC组比较,107、108、109和1010 CFU·mL-1罗伊氏乳杆菌组Caco-2细胞中病毒滴度均明显降低(P<0.01)。与对照组比较,Ab-NC组、Ab-RV组和Ab-Lac-RV组乳鼠肠道菌群菌落数量均明显减少,乳鼠肠道菌群耗竭成功。灌胃第2和4天,与RV组比较,Ab-RV组乳鼠粪便中RV VP6基因拷贝数明显降低(P<0.05);灌胃第4、6、8和10天,与Ab-RV组比较,Ab-Lac-RV组乳鼠粪便中RV VP6基因拷贝数明显降低(P<0.05)。与对照组比较,Ab-RV组和Ab-Lac-RV组乳鼠结肠组织中IL-1β、IL-10、IFN-γ及TNF-α mRNA表达水平均明显升高(P<0.05或P<0.01),IL-8 mRNA表达水平均明显降低(P<0.05),Ab-Lac-RV组乳鼠结肠组织中IL-10 mRNA表达水平明显升高(P<0.01)。 结论 罗伊氏乳杆菌可能通过上调IL-1β、IL-10、IFN-γ和TNF-α mRNA表达及下调IL-8 mRNA表达,抑制RV复制。
中图分类号:
- R373.2