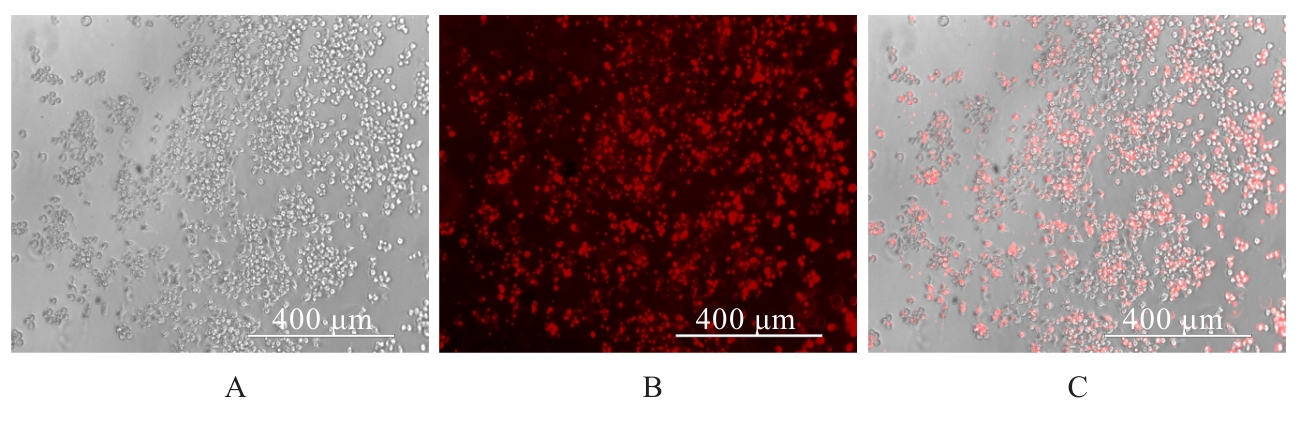
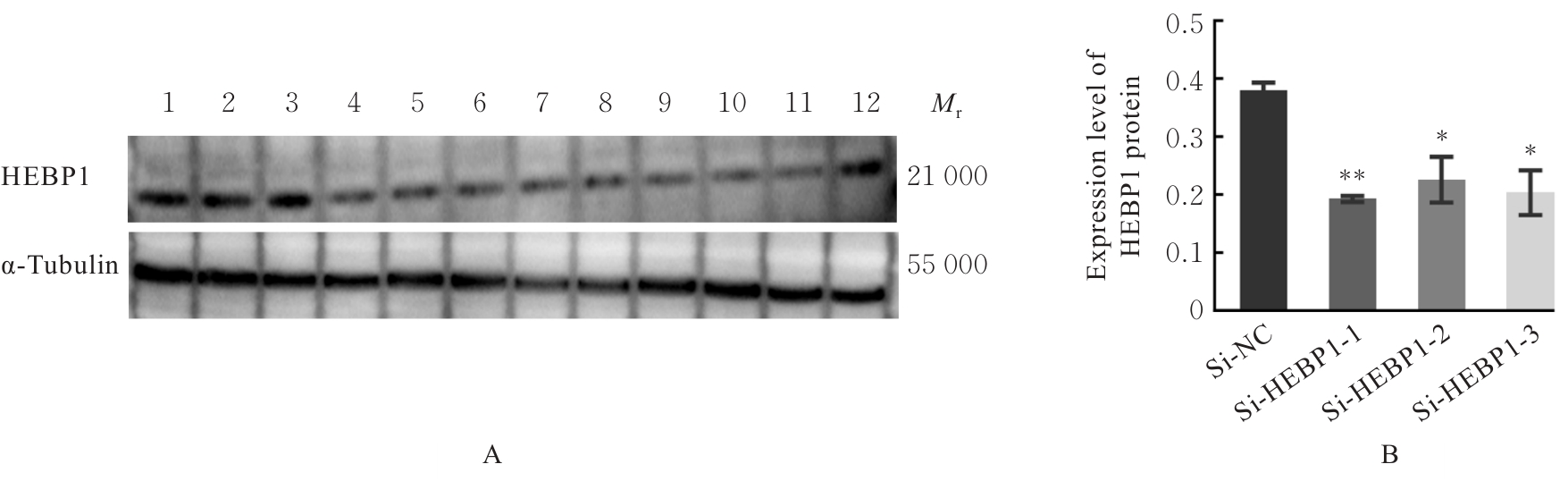
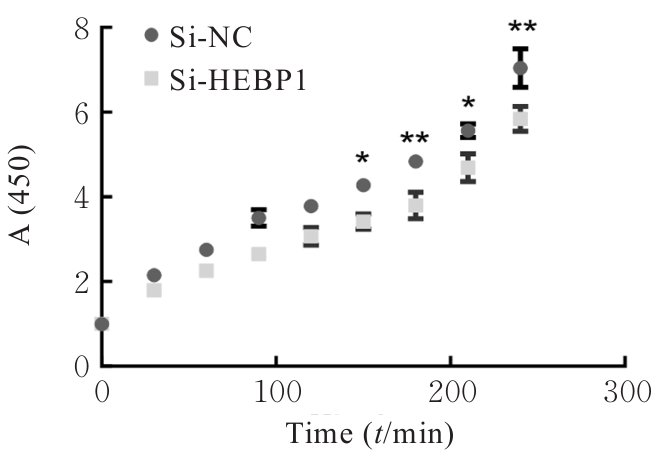
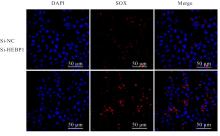
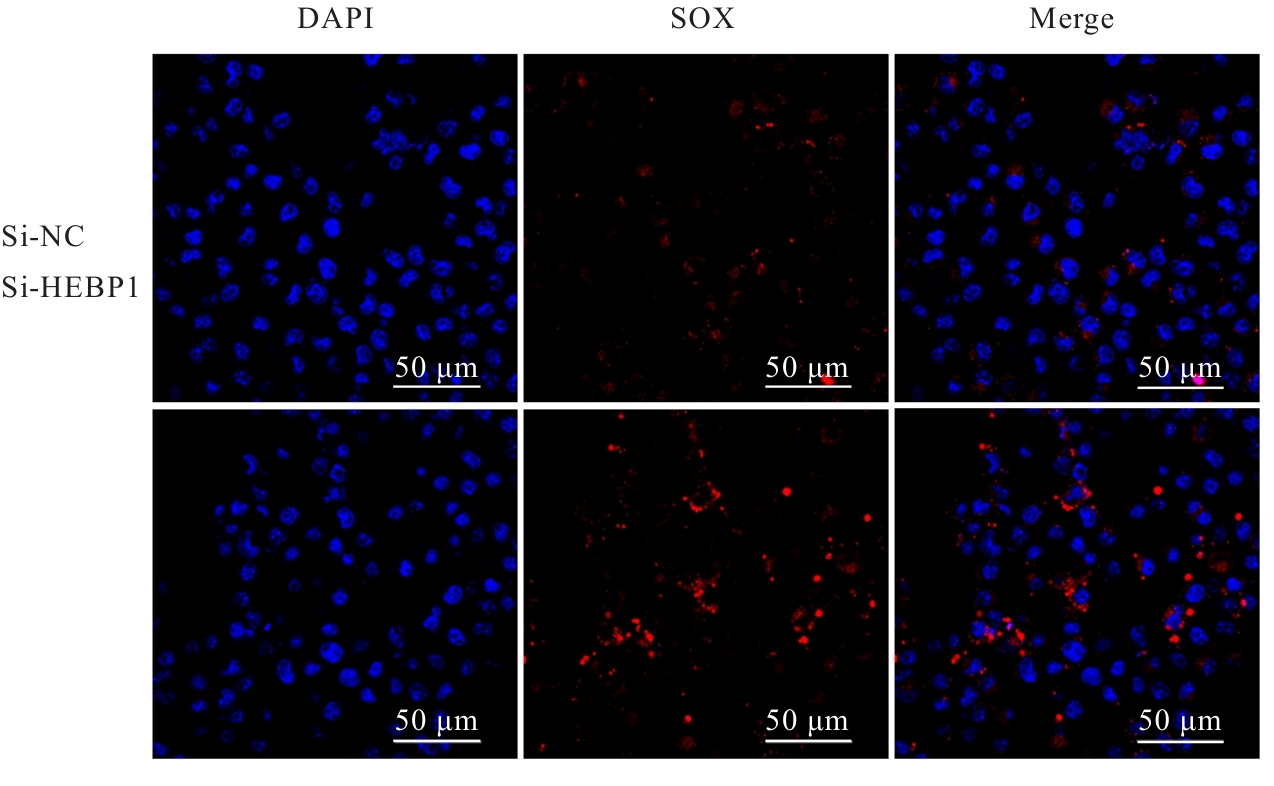
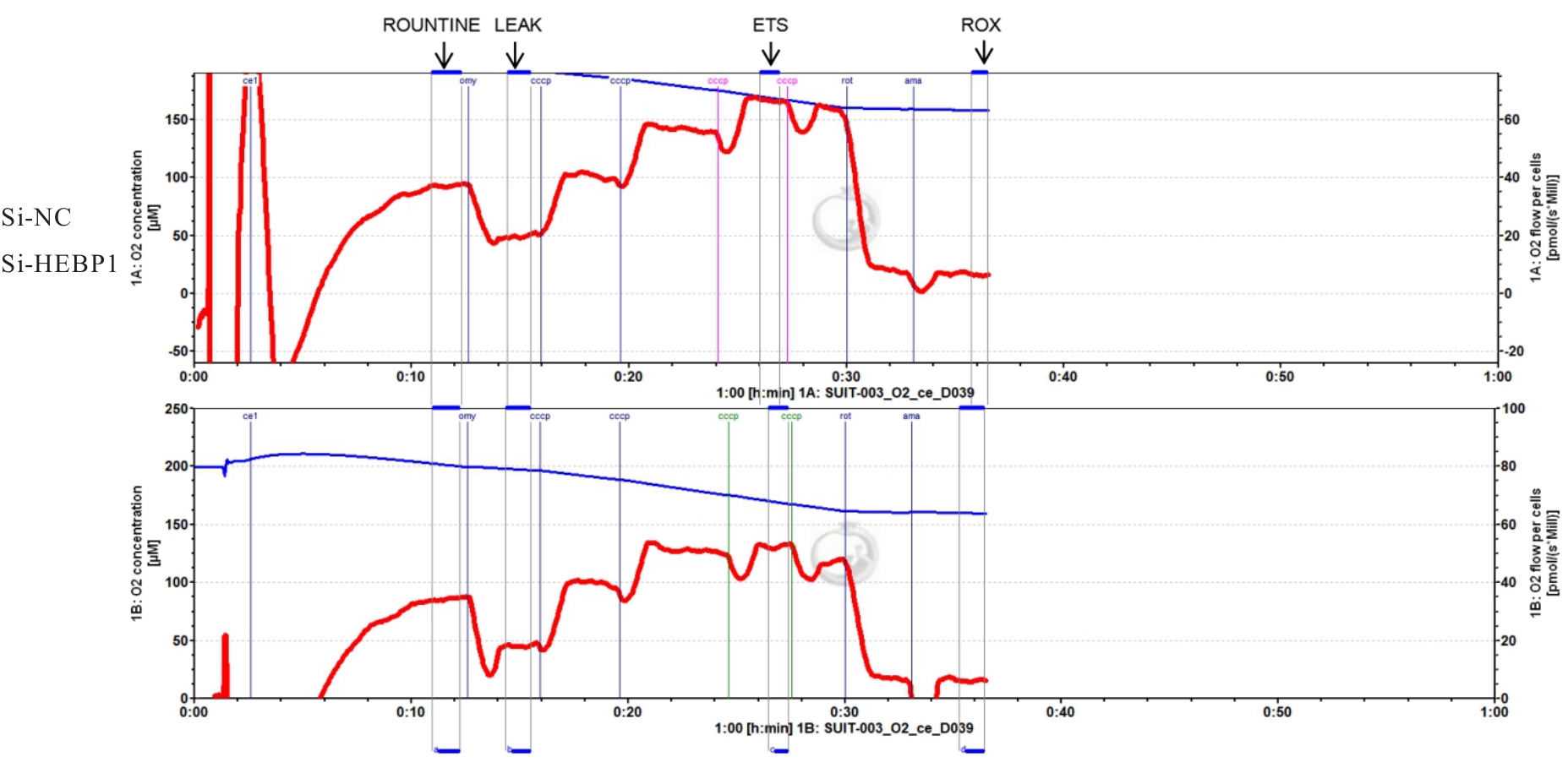
| [1] |
YAGENSKY O, KOHANSAL-NODEHI M, GUNASEELAN S, et al. Increased expression of heme-binding protein 1 early in Alzheimer’s disease is linked to neurotoxicity[J]. eLife, 2019, 8: e47498.
|
| [2] |
GOODFELLOW B J, FREIRE F, CARVALHO A L, et al. The SOUL family of heme-binding proteins: Structure and function 15 years later[J]. Coord Chem Rev, 2021, 448: 214189.
|
| [3] |
CHUA J J E. HEBP1 - An early trigger for neuronal cell death and circuit dysfunction in Alzheimer’s disease[J]. Semin Cell Dev Biol, 2023, 139: 102-110.
|
| [4] |
YIN G N. Pericyte-derived heme-binding protein 1 promotes angiogenesis and improves erectile function in diabetic mice[J]. Investig Clin Urol, 2022, 63(4): 464-474.
|
| [5] |
NICKEL W, RABOUILLE C. Mechanisms of regulated unconventional protein secretion[J]. Nat Rev Mol Cell Biol, 2009, 10(2): 148-155.
|
| [6] |
DEVOSSE T, DUTOIT R, MIGEOTTE I, et al. Processing of HEBP1 by cathepsin D gives rise to F2L, the agonist of formyl peptide receptor 3[J]. J Immunol, 2011, 187(3): 1475-1485.
|
| [7] |
GAO J L, GUILLABERT A, HU J Y, et al. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor[J]. J Immunol, 2007, 178(3): 1450-1456.
|
| [8] |
MIGEOTTE I, RIBOLDI E, FRANSSEN J D, et al. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2[J]. J Exp Med, 2005, 201(1): 83-93.
|
| [9] |
CHEN K Q, IRIBARREN P, HU J Y, et al. Activation of Toll-like receptor 2 on microglia promotes cell uptake of Alzheimer disease-associated amyloid beta peptide[J]. J Biol Chem, 2006, 281(6): 3651-3659.
|
| [10] |
CUI Y H, LE Y Y, GONG W H, et al. Bacterial lipopolysaccharide selectively up-regulates the function of the chemotactic peptide receptor formyl peptide receptor 2 in murine microglial cells[J]. J Immunol, 2002, 168(1): 434-442.
|
| [11] |
CUI Y H, LE Y, ZHANG X, et al. Up-regulation of FPR2, a chemotactic receptor for amyloid beta 1-42 (a beta 42), in murine microglial cells by TNF alpha[J]. Neurobiol Dis, 2002, 10(3): 366-377.
|
| [12] |
GOMEZ PERDIGUERO E, KLAPPROTH K, SCHULZ C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors[J]. Nature, 2015, 518(7540): 547-551.
|
| [13] |
BOTELLA LUCENA P, HENEKA M T. Inflammatory aspects of Alzheimer’s disease[J]. Acta Neuropathol, 2024, 148(1): 31.
|
| [14] |
FROST J L, SCHAFER D P. Microglia: architects of the developing nervous system[J]. Trends Cell Biol, 2016, 26(8): 587-597.
|
| [15] |
SCHAFER D P, LEHRMAN E K, KAUTZMAN A G, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner[J]. Neuron, 2012, 74(4): 691-705.
|
| [16] |
CAI Y L, LIU J L, WANG B, et al. Microglia in the neuroinflammatory pathogenesis of Alzheimer’s disease and related therapeutic targets[J]. Front Immunol, 2022, 13: 856376.
|
| [17] |
HANISCH U K, KETTENMANN H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain[J]. Nat Neurosci, 2007, 10(11): 1387-1394.
|
| [18] |
NAYAK D, ROTH T L, MCGAVERN D B. Microglia development and function[J]. Annu Rev Immunol, 2014, 32: 367-402.
|
| [19] |
XIE Z, MENG J, WU Z, et al. The dual nature of microglia in Alzheimer’s disease: a microglia-neuron crosstalk perspective[J]. Neuroscientist, 2023, 29(5): 616-638.
|
| [20] |
YAN H Y, WANG W, CUI T T, et al. Advances in the understanding of the correlation between neuroinflammation and microglia in Alzheimer’s disease[J]. Immunotargets Ther, 2024, 13: 287-304.
|
| [21] |
CALSOLARO V, EDISON P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions[J]. Alzheimers Dement, 2016, 12(6): 719-732.
|
| [22] |
TWAROWSKI B, HERBET M. Inflammatory processes in Alzheimer’s disease-pathomechanism, diagnosis and treatment: a review[J]. Int J Mol Sci, 2023, 24(7): 6518.
|
| [23] |
FITZGERALD K A, KAGAN J C. Toll-like receptors and the control of immunity[J]. Cell, 2020, 180(6): 1044-1066.
|
| [24] |
NABI S U, KHAN A, SIDDIQUI E M, et al. Mechanisms of mitochondrial malfunction in Alzheimer’s disease: new therapeutic hope[J]. Oxid Med Cell Longev, 2022, 2022: 4759963.
|
| [25] |
LI Y, XIA X H, WANG Y, et al. Mitochondrial dysfunction in microglia: a novel perspective for pathogenesis of Alzheimer’s disease[J]. J Neuroinflammation, 2022, 19(1): 248.
|
| [26] |
JOHANNSEN D L, RAVUSSIN E. The role of mitochondria in health and disease[J]. Curr Opin Pharmacol, 2009, 9(6): 780-786.
|
| [27] |
KAMATHAM P T, SHUKLA R, KHATRI D K, et al. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier[J]. Ageing Res Rev, 2024, 101: 102481.
|
| [28] |
WANG L X, PAVLOU S, DU X, et al. Glucose transporter 1 critically controls microglial activation through facilitating glycolysis[J]. Mol Neurodegener, 2019, 14(1): 2.
|
| [29] |
PRADEEPKIRAN J A, REDDY P H. Defective mitophagy in Alzheimer’s disease[J]. Ageing Res Rev, 2020, 64: 101191.
|
 )
)