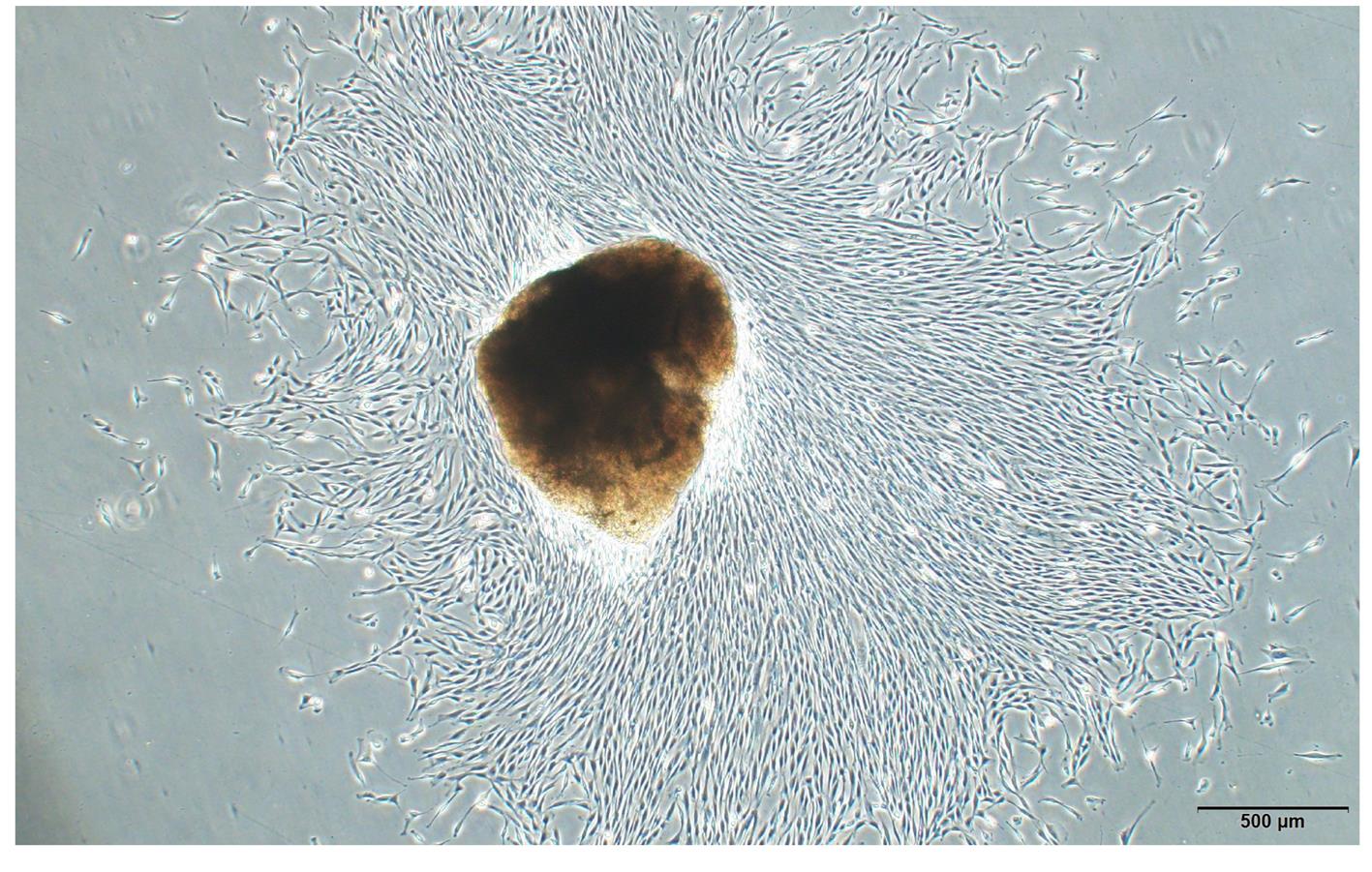
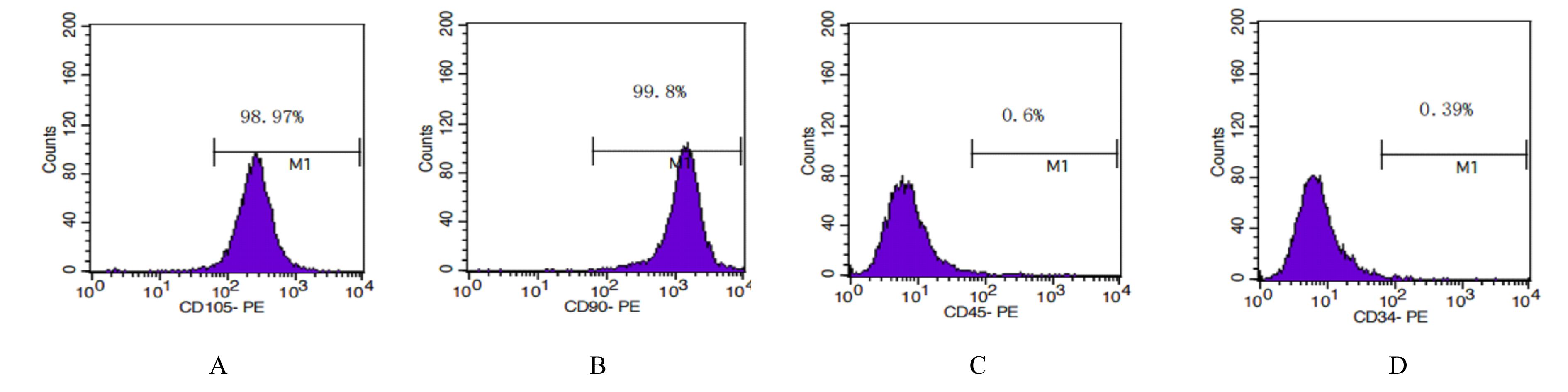
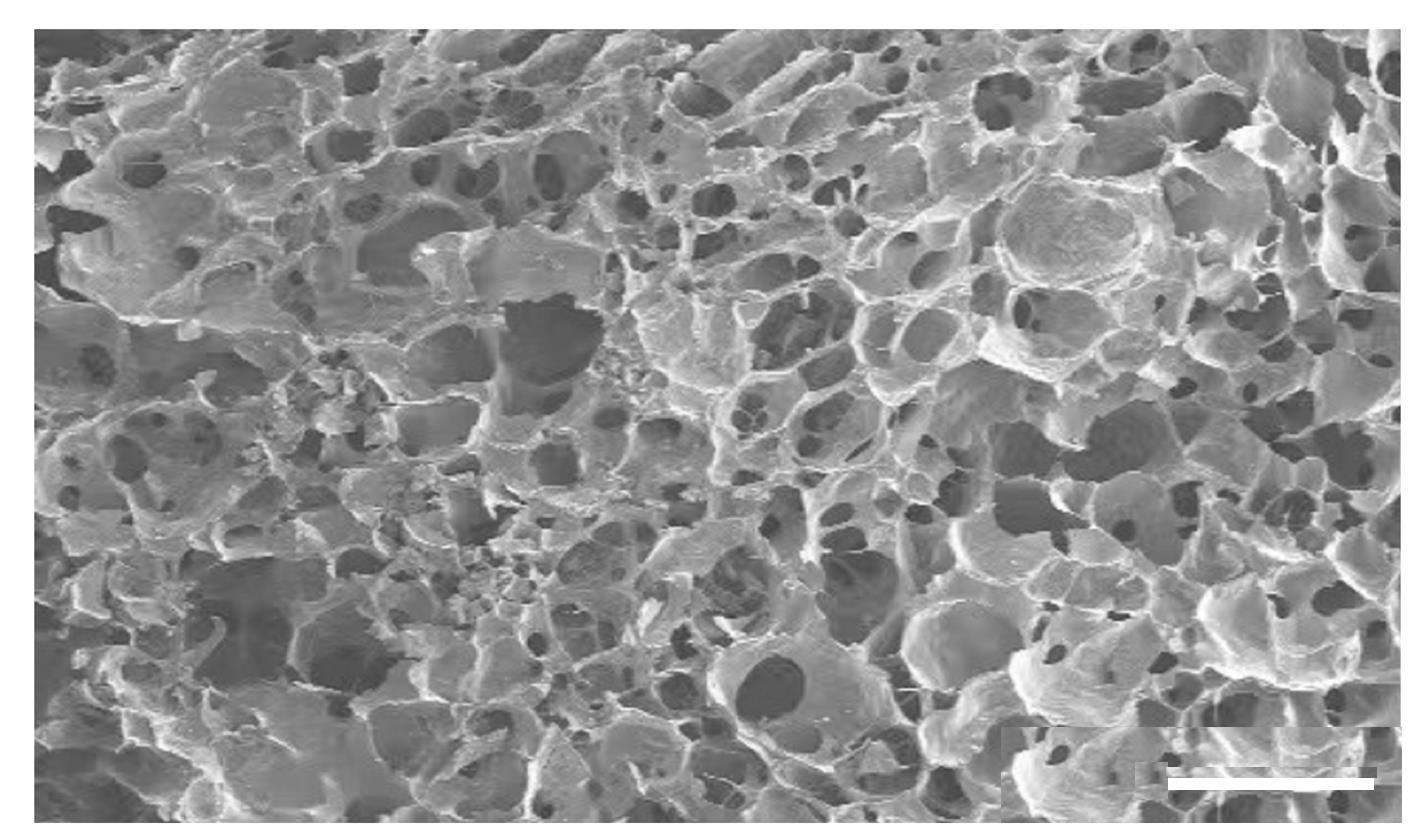
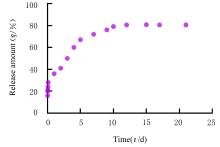
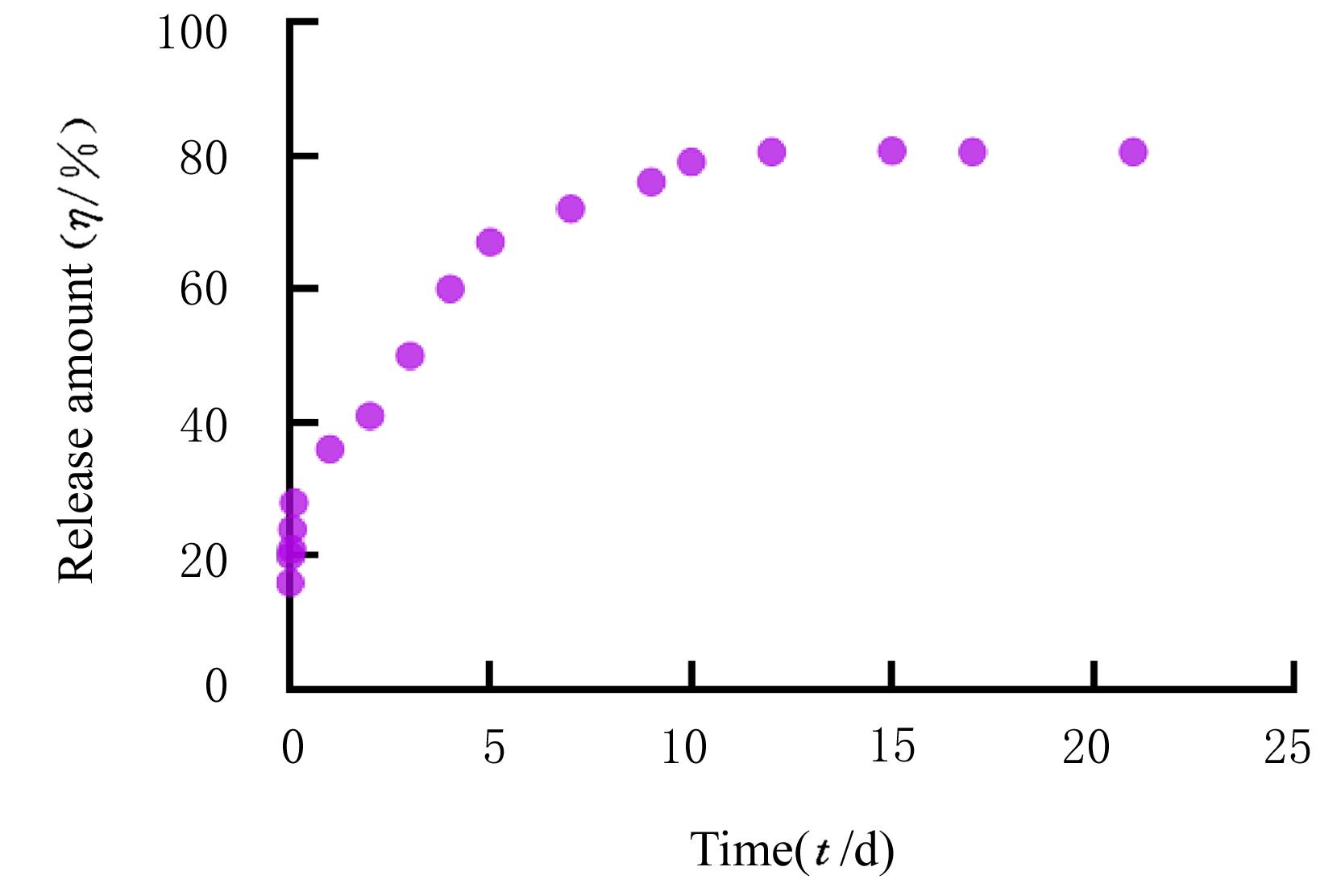
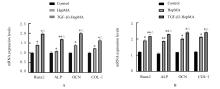
| 1 |
ADAMIČKA M, ADAMIČKOVÁ A, DANIŠOVIČ L,et al. Pharmacological approaches and regeneration of bone defects with dental pulp stem cells[J]. Stem Cells Int, 2021, 2021: 4593322.
|
| 2 |
KHARE D, BASU B, DUBEY A K. Electrical stimulation and piezoelectric biomaterials for bone tissue engineering applications[J]. Biomaterials, 2020, 258: 120280.
|
| 3 |
DU X Y, FU S Y, ZHU Y F. 3D printing of ceramic-based scaffolds for bone tissue engineering: an overview[J]. J Mater Chem B, 2018,6(27): 4397-4412.
|
| 4 |
GHASSEMI T, SHAHROODI A, EBRAHIMZADEH M H,et al. Current concepts in scaffolding for bone tissue engineering[J]. Arch Bone Jt Surg, 2018, 6(2): 90-99.
|
| 5 |
GRONTHOS S, MANKANI M, BRAHIM J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo [J]. Proc Natl Acad Sci U S A, 2000, 97(25): 13625-13630.
|
| 6 |
SUI B D, WU D, XIANG L, et al. Dental pulp stem cells: from discovery to clinical application[J]. J Endod, 2020, 46(9S): S46-S55.
|
| 7 |
LIN J J, WANG L, LIN J H, et al. Dual delivery of TGF-β3 and ghrelin in microsphere/hydrogel systems for cartilage regeneration[J].Molecules,2021,26(19):5732.
|
| 8 |
PENG Y F, TELLIER L E, TEMENOFF J S. Heparin-based hydrogels with tunable sulfation & degradation for anti-inflammatory small molecule delivery[J]. Biomater Sci, 2016, 4(9): 1371-1380.
|
| 9 |
HE C, JI H F, QIAN Y H, et al. Heparin-based and heparin-inspired hydrogels: size-effect, gelation and biomedical applications[J]. J Mater Chem B, 2019,7(8): 1186-1208.
|
| 10 |
GOLUBOVSKY J L, EJIKEME T, WINKELMAN R,et al. Osteobiologics[J].Oper Neurosurg, 2021,21(): S2-S9.
|
| 11 |
GARCÍA-GARETA E, COATHUP M J, BLUNN G W.Osteoinduction of bone grafting materials for bone repair and regeneration[J]. Bone, 2015, 81: 112-121.
|
| 12 |
NOORI A, ASHRAFI S J, VAEZ-GHAEMI R, et al. A review of fibrin and fibrin composites for bone tissue engineering[J].Int J Nanomedicine,2017,12:4937-4961.
|
| 13 |
LEE Y C, CHAN Y H, HSIEH S C, et al. Comparing the osteogenic potentials and bone regeneration capacities of bone marrow and dental pulp mesenchymal stem cells in a rabbit calvarial bone defect model[J]. Int J Mol Sci, 2019, 20(20): E5015.
|
| 14 |
ANITUA E, TROYA M, ZALDUENDO M. Progress in the use of dental pulp stem cells in regenerative medicine[J]. Cytotherapy, 2018, 20(4): 479-498.
|
| 15 |
MORIKAWA M, DERYNCK R, MIYAZONO K. TGF-β and the TGF-β family: context-dependent roles in cell and tissue physiology[J]. Cold Spring Harb Perspect Biol, 2016, 8(5): a021873.
|
| 16 |
LICHTMAN M K, OTERO-VINAS M, FALANGA V.Transforming growth factor beta (TGF-β) isoforms in wound healing and fibrosis[J].Wound Repair Regen,2016,24(2):215-222.
|
| 17 |
KOMAI T, OKAMURA T, INOUE M, et al. Reevaluation of pluripotent cytokine TGF-β3 in immunity[J]. Int J Mol Sci, 2018, 19(8): E2261.
|
| 18 |
LI Y F, QIAO Z F, YU F L, et al. Transforming growth factor-β3/chitosan sponge (TGF-β3/CS) facilitates osteogenic differentiation of human periodontal ligament stem cells[J].Int J Mol Sci,2019,20(20): E4982.
|
| 19 |
KIM M, KIM S E, KANG S S, et al. The use of de-differentiated chondrocytes delivered by a heparin-based hydrogel to regenerate cartilage in partial-thickness defects[J]. Biomaterials, 2011, 32(31): 7883-7896.
|
| 20 |
HSU F M, HU M H, JIANG Y S, et al. Antibacterial polypeptide/heparin composite hydrogels carrying growth factor for wound healing[J]. Mater Sci Eng C Mater Biol Appl, 2020, 112: 110923.
|
 )
)