| 1 |
ROBICHAUX J P, LE X N, VIJAYAN R S K, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC[J]. Nature, 2021, 597(7878): 732-737.
|
| 2 |
KOHNO T, MATSUI T, ENATSU S. Differences between EGFR exon 19 deletion and exon 21 L858R point mutation, frequently detected EGFR mutations in patients with non-small cell lung cancer, from a molecular biology viewpoint[J]. Gan To Kagaku Ryoho, 2021, 48(12): 1463-1467.
|
| 3 |
SIGISMUND S, AVANZATO D, LANZETTI L. Emerging functions of the EGFR in cancer[J]. Mol Oncol, 2018, 12(1): 3-20.
|
| 4 |
NGUYEN P V, ALLARD-VANNIER E, CHOURPA I,et al. Nanomedicines functionalized with anti-EGFR ligands for active targeting in cancer therapy: biological strategy, design and quality control[J]. Int J Pharm, 2021, 605: 120795.
|
| 5 |
URIBE M L, MARROCCO I, YARDEN Y. EGFR in cancer: signaling mechanisms, drugs, and acquired resistance[J]. Cancers, 2021, 13(11): 2748.
|
| 6 |
LEE Y J, HO S R, GRAVES J D, et al. CGRRF1, a growth suppressor, regulates EGFR ubiquitination in breast cancer[J]. Breast Cancer Res, 2019, 21(1): 134.
|
| 7 |
VAUGHAN R M, KUPAI A, ROTHBART S B. Chromatin regulation through ubiquitin and ubiquitin-like histone modifications[J]. Trends Biochem Sci, 2021, 46(4): 258-269.
|
| 8 |
DAMGAARD R B. The ubiquitin system: from cell signalling to disease biology and new therapeutic opportunities[J].Cell Death Differ,2021,28(2):423-426.
|
| 9 |
RODRIGUEZ CARVAJAL A, GRISHKOVSKAYA I, GOMEZ DIAZ C, et al. The linear ubiquitin chain assembly complex (LUBAC) generates heterotypic ubiquitin chains[J]. Elife, 2021, 10: e60660.
|
| 10 |
LIU J P, WANG Y L, GONG Y K, et al. Structural insights into SHARPIN-mediated activation of HOIP for the linear ubiquitin chain assembly[J]. Cell Rep, 2017, 21(1): 27-36.
|
| 11 |
DRABER P, KUPKA S, REICHERT M, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes[J]. Cell Rep, 2015, 13(10): 2258-2272.
|
| 12 |
JIMBO K, KOIDE S, ITO T, et al. Deficiency of the E3 ligase hoip impairs adult hematopoiesis and myeloid leukemia[J]. Blood, 2021, 138: 1153.
|
| 13 |
ELLIOTT P R, LESKE D, HRDINKA M, et al. SPATA2 links CYLD to LUBAC, activates CYLD, and controls LUBAC signaling[J].Mol Cell,2016,63(6): 990-1005.
|
| 14 |
TAKIUCHI T, NAKAGAWA T, TAMIYA H, et al. Suppression of LUBAC-mediated linear ubiquitination by a specific interaction between LUBAC and the deubiquitinases CYLD and OTULIN[J]. Genes Cells, 2014, 19(3): 254-272.
|
| 15 |
SCHAEFFER V, AKUTSU M, OLMA M H, et al. Binding of OTULIN to the PUB domain of HOIP controls NF-κB signaling[J]. Mol Cell, 2014, 54(3): 349-361.
|
| 16 |
DOUGLAS T, SALEH M. The plot thickens: OTULIN regulation in cell death[J]. Mol Cell Oncol, 2020, 7(4): 1740541.
|
| 17 |
RITTINGER K, IKEDA F. Linear ubiquitin chains: enzymes, mechanisms and biology[J]. Open Biol, 2017, 7(4): 170026.
|
| 18 |
BURNETT H, EMICH H, CARROLL C, et al. Epidemiological and clinical burden of EGFR Exon 20 insertion in advanced non-small cell lung cancer: a systematic literature review[J].PLoS One,2021,16(3): e0247620.
|
| 19 |
PONSIOEN B, POST J B, BUISSANT DES AMORIE J R, et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling[J]. Nat Cell Biol, 2021, 23(4): 377-390.
|
| 20 |
JIANG Y H, LIM J, WU K C, et al. PAR2 induces ovarian cancer cell motility by merging three signalling pathways to transactivate EGFR[J]. Br J Pharmacol, 2021, 178(4): 913-932.
|
| 21 |
LI T, TAO Z H, ZHU Y H, et al. Exosomal annexin A6 induces gemcitabine resistance by inhibiting ubiquitination and degradation of EGFR in triple-negative breast cancer[J].Cell Death Dis,2021,12(7): 684.
|
| 22 |
CHEN N, HAN X D, YIN B, et al. FGD5 facilitates tumor growth by regulating EGFR ubiquitination in gastric cancer[J]. Biochem Biophys Res Commun, 2021, 562: 43-49.
|
| 23 |
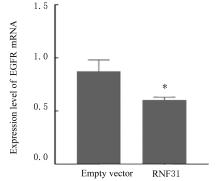
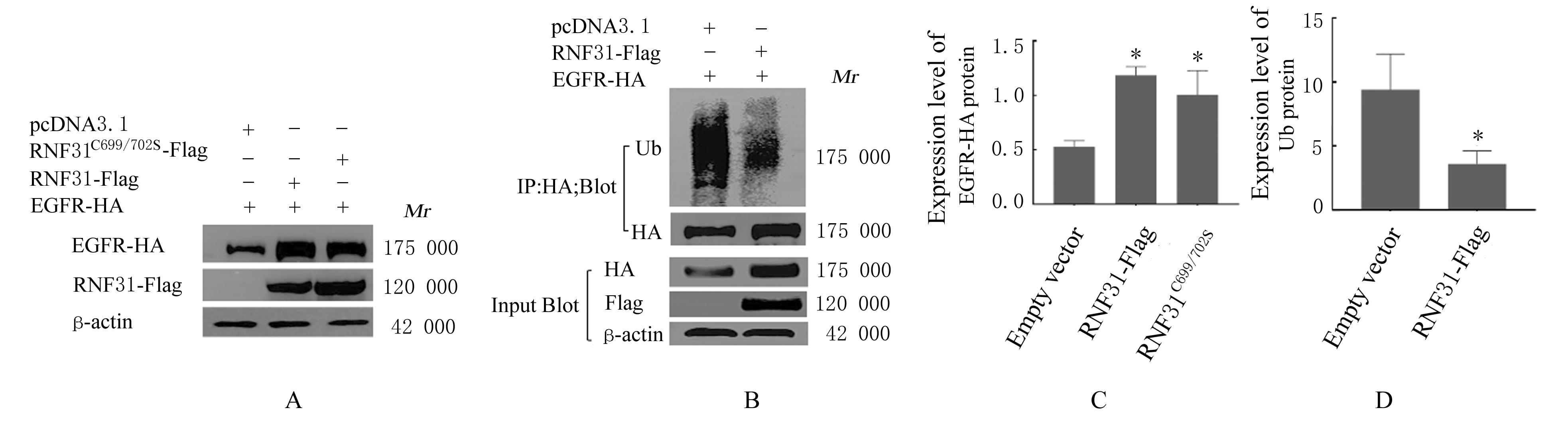
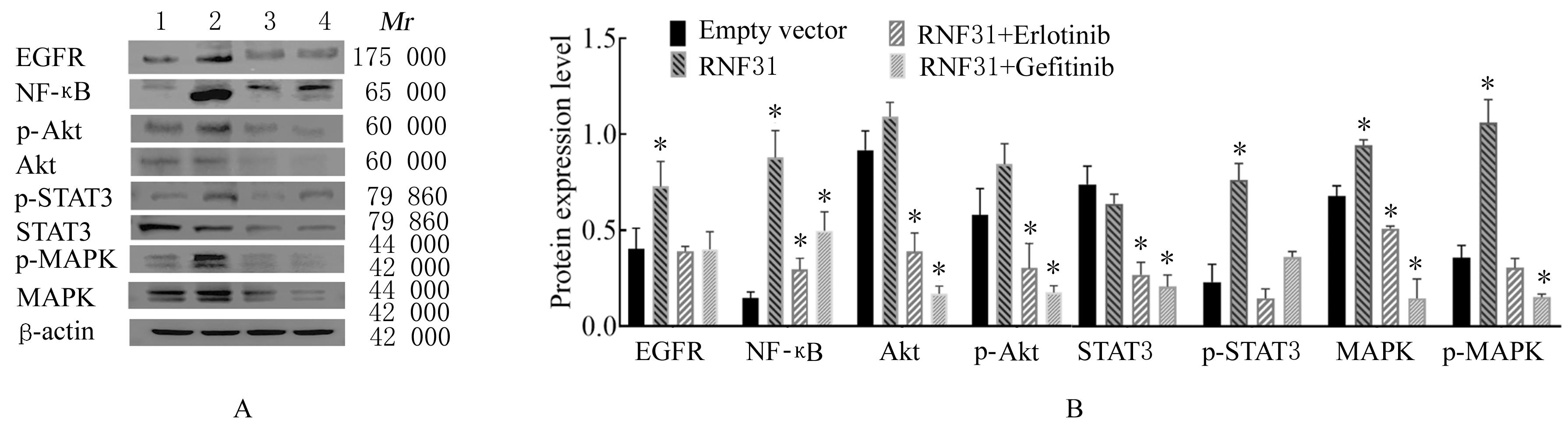
LI S, ZHENG X M, HU Y C, et al. RNF31 mediated ubiquitination of A20 aggravates inflammation and hepatocyte apoptosis through the TLR4/MyD88/NF-κB signaling pathway[J]. Chem Biol Interact, 2021, 348: 109623.
|
| 24 |
ZHU J, ZHAO C, KHARMAN-BIZ A, et al. Correction: The atypical ubiquitin ligase RNF31 stabilizes estrogen receptor α and modulates estrogen-stimulated breast cancer cell proliferation[J]. Oncogene, 2019, 38(2): 299-300.
|
| 25 |
NIU Z G, LI X, DONG S X, et al. The E3 ubiquitin ligase HOIP inhibits cancer cell apoptosis via modulating PTEN stability[J]. J Cancer, 2021, 12(21):6553-6562.
|
| 26 |
ELLIOTT P R, NIELSEN S V, MARCO-CASANOVA P, et al. Molecular basis and regulation of OTULIN-LUBAC interaction[J].Mol Cell, 2014, 54(3): 335-348.
|
| 27 |
STANGL A, ELLIOTT P R, PINTO-FERNANDEZ A,et al. Regulation of the endosomal SNX27-retromer by OTULIN[J]. Nat Commun, 2019, 10(1): 4320.
|
 )
)