Journal of Jilin University(Medicine Edition) ›› 2022, Vol. 48 ›› Issue (6): 1455-1461.doi: 10.13481/j.1671-587X.20220611
• Research in basic medicine • Previous Articles Next Articles
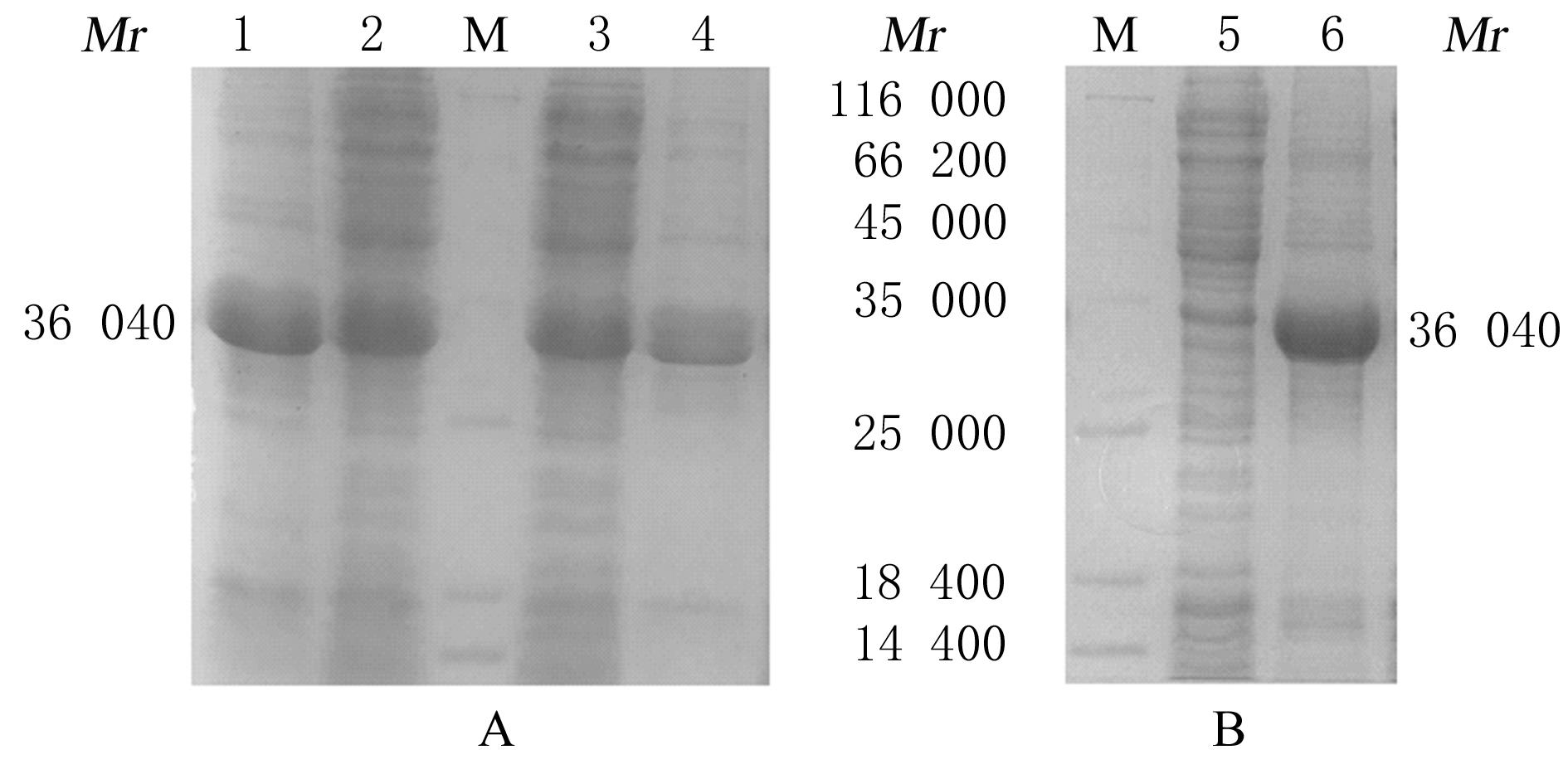
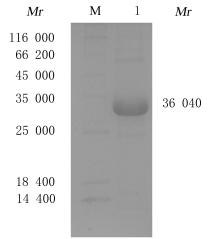
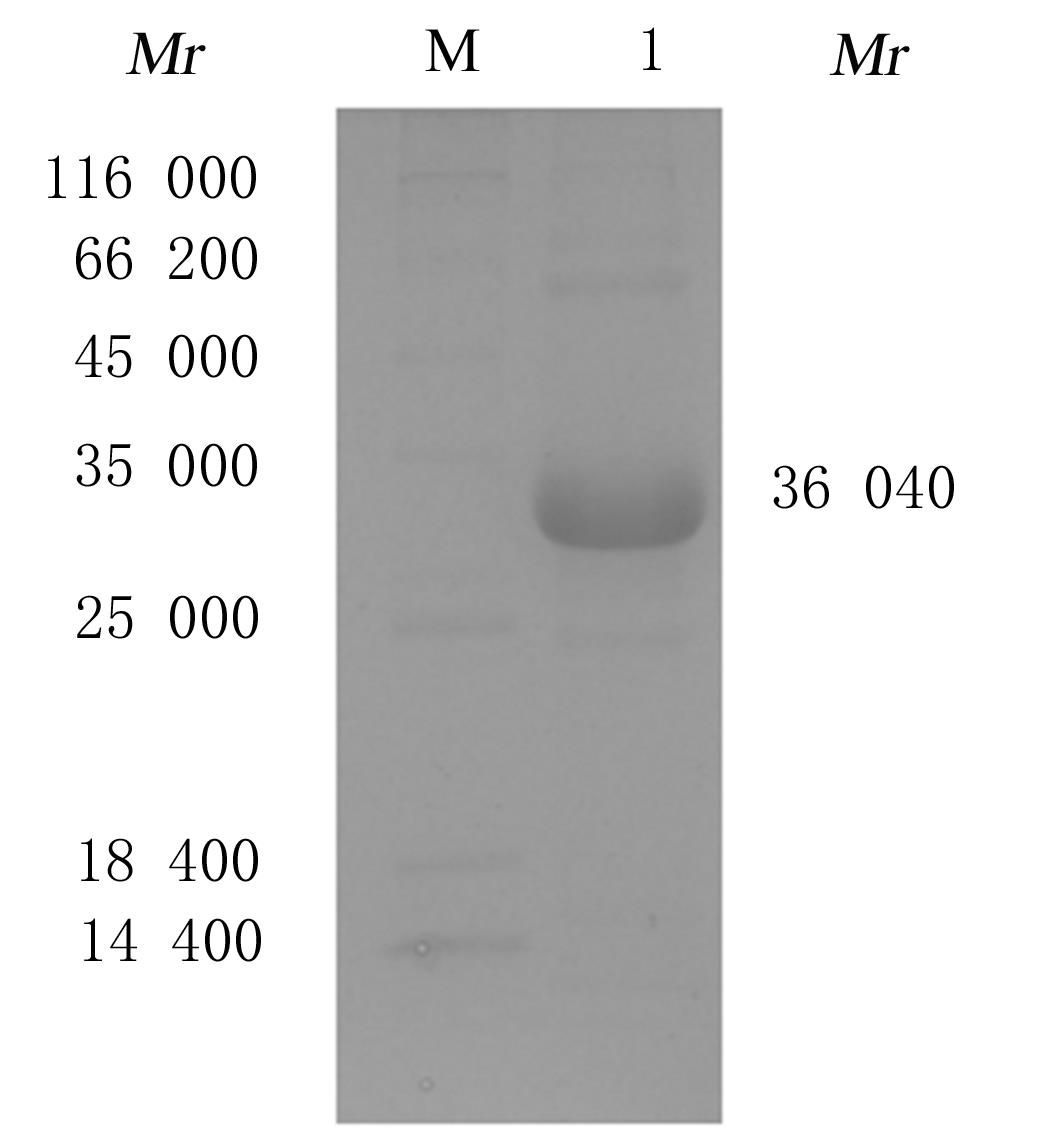
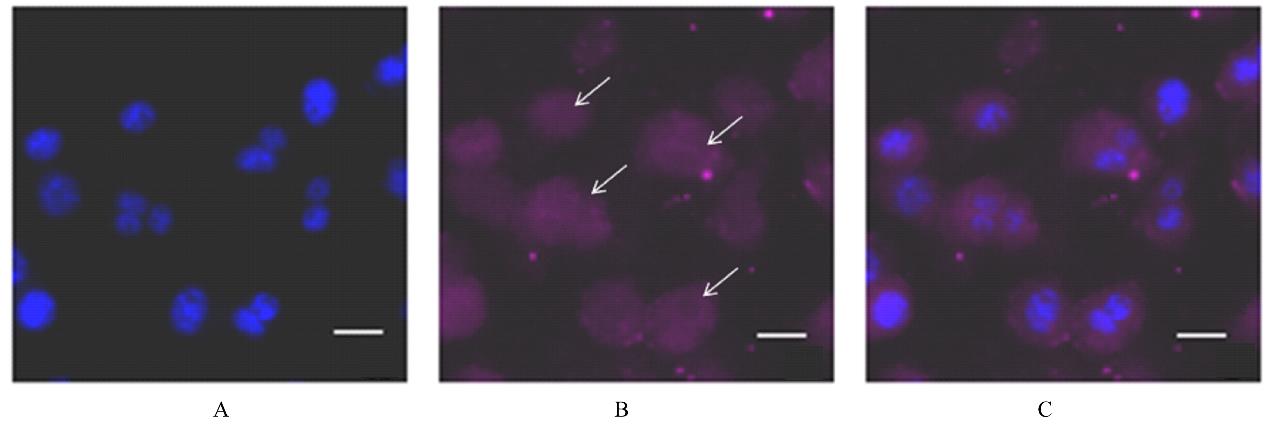
Preparation of tumor homing peptides-near-infrared fluorescent protein miRFP670-LyP1 fusion protein and its fluorescence characteristics
Fuxu YANG1,Nannan HU1,Chong GUO1,Yeteng MU1,Han XUE1,Yuxin FAN1,Fenglin GUO1,Xingang GUAN1,2( )
)
- 1.Key Laboratory of Pharmaceutics and Bioengineering,School of Medical Technology,Beihua University,Jilin 132013,China
2.Department of Basic Medicine,College of Medical Sciences,Taizhou University,Taizhou 318000,China
-
Received:2022-02-11Online:2022-11-28Published:2022-12-07 -
Contact:Xingang GUAN E-mail:guanxg@ciac.ac.cn
CLC Number:
- R394.3
Cite this article
Fuxu YANG,Nannan HU,Chong GUO,Yeteng MU,Han XUE,Yuxin FAN,Fenglin GUO,Xingang GUAN. Preparation of tumor homing peptides-near-infrared fluorescent protein miRFP670-LyP1 fusion protein and its fluorescence characteristics[J].Journal of Jilin University(Medicine Edition), 2022, 48(6): 1455-1461.
share this article
| 1 | WEISSLEDER R. A clearer vision for in vivo imaging[J]. Nat Biotechnol, 2001, 19(4): 316-317. |
| 2 | HALL C, VON GRABOWIECKI Y, PEARCE S P, et al. iRFP (near-infrared fluorescent protein) imaging of subcutaneous and deep tissue tumours in mice highlights differences between imaging platforms[J]. Cancer Cell Int, 2021, 21(1): 247. |
| 3 | FILONOV G S, PIATKEVICH K D, TING L M,et al. Bright and stable near-infrared fluorescent protein for in vivo imaging[J]. Nat Biotechnol, 2011,29(8): 757-761. |
| 4 | 关新刚, 苏维恒, 于 欣, 等. Tat-GFP融合蛋白的表达纯化及其穿膜活性[J]. 吉林大学学报(医学版), 2014, 40(4): 725-728. |
| 5 | 刘珊珊, 彭 亮, 陈建林, 等. 红色荧光蛋白mCherry在衣原体感染模型中的应用[J]. 中华微生物学和免疫学杂志, 2020, 40(12): 950-957. |
| 6 | SHCHERBAKOVA D M, VERKHUSHA V V. Near-infrared fluorescent proteins for multicolor in vivo imaging[J]. Nat Methods, 2013, 10(8): 751-754. |
| 7 | PIATKEVICH K D, SUBACH F V, VERKHUSHA V V.Far-red light photoactivatable near-infrared fluorescent proteins engineered from a bacterial phytochrome[J]. Nat Commun, 2013, 4: 2153. |
| 8 | SHCHERBAKOVA D M, BALOBAN M, EMELYANOV A V, et al. Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging[J]. Nat Commun, 2016, 7: 12405. |
| 9 | AN F F, CHEN N D, CONLON W J, et al. Small ultra-red fluorescent protein nanoparticles as exogenous probes for noninvasive tumor imaging in vivo [J]. Int J Biol Macromol, 2020, 153: 100-106. |
| 10 | HERBERT F C, BROHLIN O R, GALBRAITH T, et al. Supramolecular encapsulation of small-ultrared fluorescent proteins in virus-like nanoparticles for noninvasive in vivo imaging agents[J]. Bioconjug Chem, 2020, 31(5): 1529-1536. |
| 11 | 吴 娇, 杨唐斌, 柳长柏. 肿瘤归巢肽及其在肿瘤靶向治疗中的应用[J]. 海南医学, 2016, 27(1): 82-84. |
| 12 | FOGAL V, ZHANG L L, KRAJEWSKI S, et al. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma[J]. Cancer Res, 2008, 68(17): 7210-7218. |
| 13 | JIANG Y J, LIU S J, ZHANG Y, et al. Magnetic mesoporous nanospheres anchored with LyP-1 as an efficient pancreatic cancer probe[J]. Biomaterials, 2017, 115: 9-18. |
| 14 | REN Y, CHEUNG H W, VON MALTZHAN G,et al. Targeted tumor-penetrating siRNA nano complexes for credentialing the ovarian cancer oncogene ID4[J]. Sci Transl Med, 2012, 4(147): 147ra112. |
| 15 | TIMUR S S, YALÇIN G, ÇEVIK Ö,et al. Molecular dynamics, thermodynamic, and mutational binding studies for tumor-specific LyP-1 in complex with p32[J]. J Biomol Struct Dyn, 2018, 36(5): 1134-1144. |
| 16 | ZHANG X Q, HUANG Y M, SONG H L, et al. Inhibition of growth and lung metastasis of breast cancer by tumor-homing triple-bioresponsive nanotherapeutics[J]. J Control Release, 2020, 328: 454-469. |
| 17 | SONG N N, ZHAO L Z, XU X Y, et al. LyP-1-modified multifunctional dendrimers for targeted antitumor and antimetastasis therapy[J]. ACS Appl Mater Interfaces, 2020, 12(11): 12395-12406. |
| 18 | HUANG X, YIN Y L, WU M, et al. LyP-1 peptide-functionalized gold nanoprisms for SERRS imaging and tumor growth suppressing by PTT induced-hyperthermia[J]. Chin Chem Lett, 2019, 30(6): 1335-1340. |
| 19 | LV C, ZHANG T Y, LIN Y, et al. Transformation of viral light particles into near-infrared fluorescence quantum dot-labeled active tumor-targeting nanovectors for drug delivery[J]. Nano Lett, 2019, 19(10): 7035-7042. |
| 20 | ZHENG F F, WANG C, MENG T T, et al. Outer-frame-degradable nanovehicles featuring near-infrared dual luminescence for in vivo tracking of protein delivery in cancer therapy[J]. ACS Nano, 2019, 13(11): 12577-12590. |
| 21 | GODARD A, KALOT G, PLIQUETT J, et al. Water-soluble aza-BODIPYs: biocompatible organic dyes for high contrast in vivo NIR-II imaging[J]. Bioconjug Chem, 2020, 31(4): 1088-1092. |
| 22 | COSCO E D, LIM I, SLETTEN E M. Photophysical properties of indocyanine green in the shortwave infrared region[J]. Chem Photo Chem, 2021, 5(8): 727-734. |
| 23 | 余 梦, 张子俊, 朱国委, 等. Ag2S基近红外Ⅱ区荧光量子点的水相合成优化探究[J]. 化学学报,2021,79(10): 1281-1285. |
| 24 | CHANG E, THEKKEK N, YU W W, et al. Evaluation of quantum dot cytotoxicity based on intracellular uptake[J]. Small, 2006, 2(12): 1412-1417. |
| 25 | RODRIGUEZ E A, TRAN G N, GROSS L A, et al. A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein[J]. Nat Methods, 2016, 13(9): 763-769. |
| 26 | RUMYANTSEV K A, SHCHERBAKOVA D M, ZAKHAROVA N I, et al. Minimal domain of bacterial phytochrome required for chromophore binding and fluorescence[J]. Sci Rep, 2015, 5: 18348. |
| 27 | WILSON A L, WILSON K L, BILANDZIC M, et al. Non-invasive fluorescent monitoring of ovarian cancer in an immunocompetent mouse model[J]. Cancers, 2018, 11(1): 32. |
| 28 | 段海潇, 杨俊寒, 王 琳, 等. 表达近红外荧光蛋白的结肠癌细胞系的建立[J]. 湖北工业大学学报, 2020, 35(5): 71-74. |
| [1] | Fengjuan JIAO,Junjie LIU,Siwei LU,Dongjun JIANG. Construction of prokaryotic expression vector of site directed mutant TFEB by SOE PCR technique and its induced expression and purification in vitro [J]. Journal of Jilin University(Medicine Edition), 2022, 48(5): 1109-1115. |
| [2] | Lili HUANG,Yuxuan LIU,Kaiyi FANG,Yeteng MU,Nannan HU,Chong GUO,Fuxu YANF,Xingang GUAN. Preparation of oxaliplatin-loaded cell membrane nanodrugs and its killing effect on colon cancer cells of mice [J]. Journal of Jilin University(Medicine Edition), 2021, 47(6): 1422-1428. |
| [3] | LI Shimeng, ZHAO Lichun, QIAO Lu, HE Chengyan, LIU Zhuo. Prokaryotic expressionof ATP5B and preparation and identification of its monoclonal antibody [J]. Journal of Jilin University(Medicine Edition), 2019, 45(01): 184-189. |
| [4] | SUN Minying, ZHOU Hongyue, JIE Jing, XIE Fei, ZHAI Ruiping, CHENTanxiu, YUAN Hongyan, TAI Guixiang. Specific Th2 immune response of MUC1 induced by thmosin α1 [J]. Journal of Jilin University Medicine Edition, 2018, 44(02): 299-304. |
| [5] | LI Huimin, YI Huanfa. Prokaryotic expression and purification of Toxoplasma gondii profilin protein [J]. Journal of Jilin University Medicine Edition, 2017, 43(06): 1109-1114. |
| [6] | HUO Yunlong, WANG Chunyu, ZHAO Yue. Subcellular localization of Mu2 protein and screening of its interacting proteins [J]. Journal of Jilin University Medicine Edition, 2016, 42(06): 1054-1058. |
| [7] | LIU Wei, YAO Yang, MA Xiaoxiao, DENG Yuxuan, MEI Di, LIU Lei, WANG Huiyan. Construction of fusion expression vector pET22b-SUMO-FGFR4 and optimization of expression conditions in E.coli [J]. Journal of Jilin University Medicine Edition, 2016, 42(04): 642-647. |
| [8] | SHAO Min, WANG Xinying, LIU Xing, WANG Yan, ZHOU Hefeng, CE Zhenglong. Construction of prokaryotic expression vector of hISO gene and its expression in E.coli and identification [J]. Journal of Jilin University Medicine Edition, 2016, 42(01): 59-63. |
| [9] | YANG Liu, WANG Xiaowan, WANG Bingyu, SONG Runmin, LI Jiang. Recombination of GST-hZimp fusion protein and preparation of anti-hZimp10 antibody [J]. Journal of Jilin University Medicine Edition, 2015, 41(03): 656-661. |
| [10] | ZHAO Jin-xia,WANG Zhi-ping,TAO Yan,HE Zhen-hua,GUO Qi,HONG Mei. Construction of human BTG2 eukaryotic expression vector with FLAG tag and its expression in HeLa cells [J]. Journal of Jilin University Medicine Edition, 2014, 40(06): 1149-1154. |
| [11] | GUAN Xin-gang,SU Wei-heng,YU Xin,TONG Hai-bin,SUN Xin. Expression and purification of Tat-GFP fusion protein and its cell membrane penetrating activity [J]. Journal of Jilin University Medicine Edition, 2014, 40(04): 725-728. |
| [12] | WANG Juan,XIE Fei,CHEN Tan-xiu,SUN Xia-xia,LI Qiong-shu,TAI Gui-xiang . Evaluation on effect of ultrafiltration technology on endotoxin removal from recombinant MUC1-MBP fusion protein [J]. Journal of Jilin University Medicine Edition, 2014, 40(03): 539-542. |
| [13] | LI Song,YANG Li-bin,ZHANG Shu-yan,JIN Yu,CHEN Xiu-hua,LI Xin,LIU Ling,LI Shu-lei,LI Shu-hong. Prokaryotic expression and identification of recombinant Cystatin of Cyprinus carpio var.Jian and preparation of its polyclonal antibody [J]. Journal of Jilin University Medicine Edition, 2014, 40(01): 204-209. |
| [14] | Suyalatu,ZHANG Yong-sheng,CHEN Yu-jie,HU Xiao-ping,WANG Li-ying,WAN Min,WU Qi-zhu. Construction of prokaryotic expression vector of recombinant mouse IFN-β and screening for solubilization conditions of inclusion body [J]. Journal of Jilin University Medicine Edition, 2013, 39(4): 841-846. |
| [15] | LIU Tong,LI Yang,LI Dan-ni*,GENG Nan-xi,LI Feng. Construction of GST-tagged prokaryotic expression plasmids of human PAK6 gene and expressions of their recombinant proteins [J]. Journal of Jilin University Medicine Edition, 2013, 39(3): 437-440. |
|