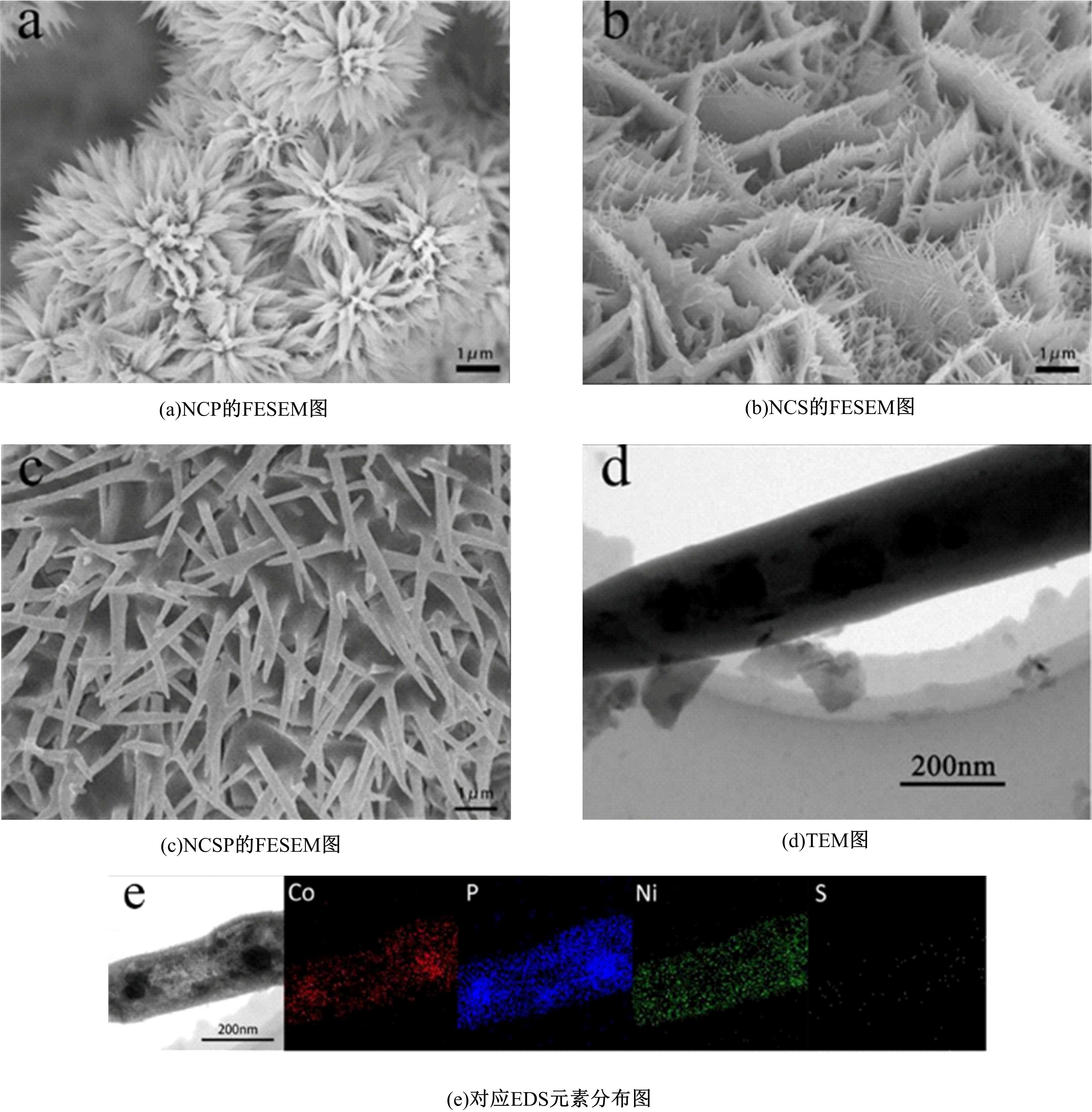
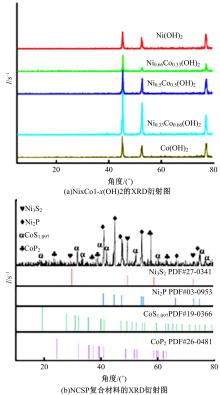
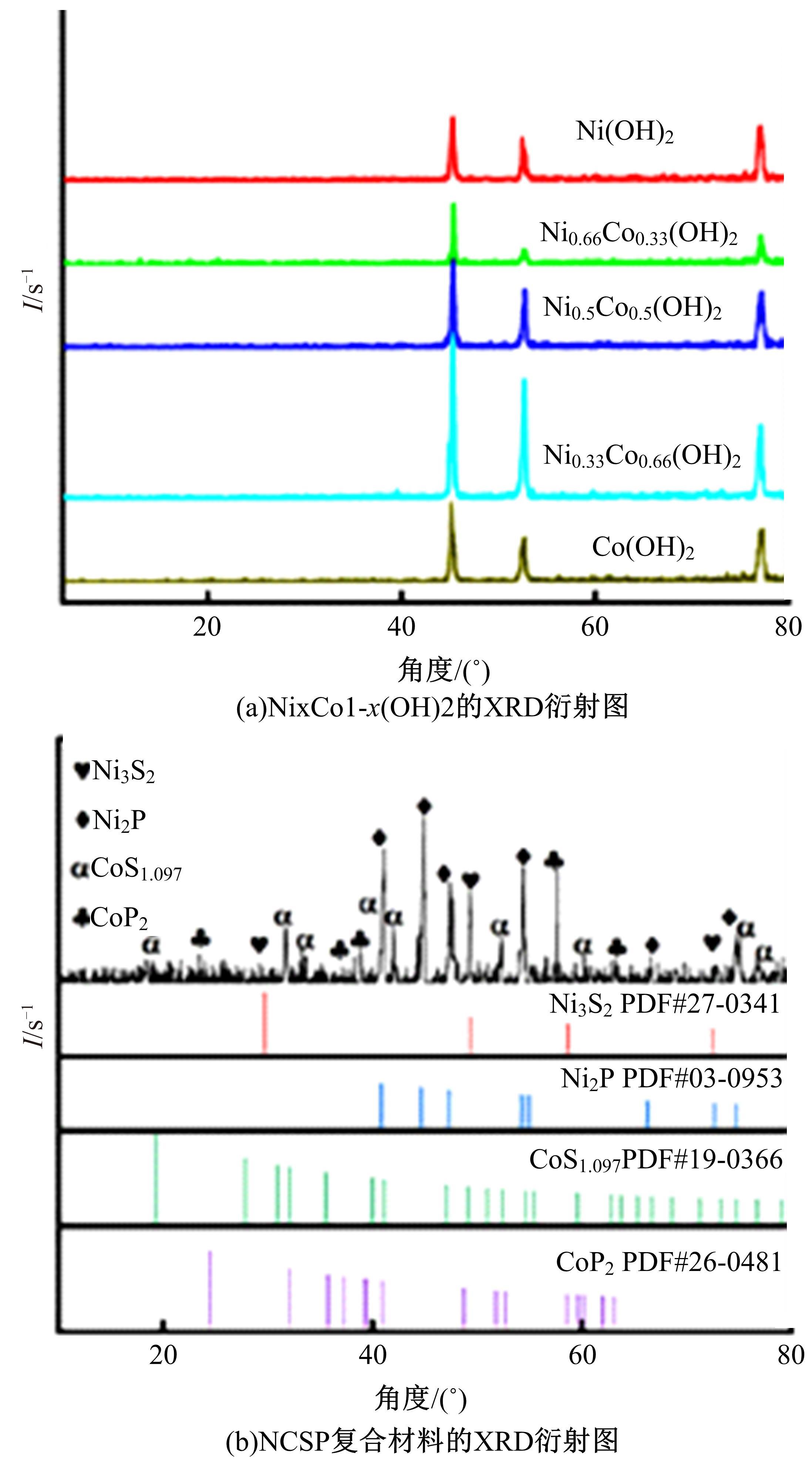
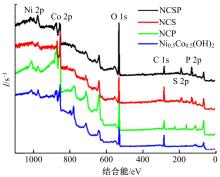
吉林大学学报(工学版) ›› 2024, Vol. 54 ›› Issue (3): 674-682.doi: 10.13229/j.cnki.jdxbgxb.20220457
• 材料科学与工程 • 上一篇
镍-钴硫/磷化物纳米复合材料用于析氢反应
魏素凤1( ),孙殿东2,张君宇3,王瑜喆3,李恩泽1,王国勇3(
),孙殿东2,张君宇3,王瑜喆3,李恩泽1,王国勇3( )
)
- 1.长春工业大学 材料科学与工程学院,长春 130012
2.鞍钢集团 海洋装备用金属材料及其应用国家重点实验室,辽宁 鞍山 114021
3.吉林大学 材料科学与工程学院,长春 130022
Nickel⁃Cobalt sulfide/phosphide nanocomposite for hydrogen evolution reaction
Su-feng WEI1( ),Dian-dong SUN2,Jun-yu ZHANG3,Yu-zhe WANG3,En-ze LI1,Guo-yong WANG3(
),Dian-dong SUN2,Jun-yu ZHANG3,Yu-zhe WANG3,En-ze LI1,Guo-yong WANG3( )
)
- 1.College of Materials Science and Engineering,Changchun University of Technology,Changchun 130012,China
2.State Key Laboratory of Metal Material for Marine Equipment and Application,Ansteel Group,Anshan 114021,China
3.College of Materials Science and Engineering,Jilin University,Changchun 130022,China
摘要:
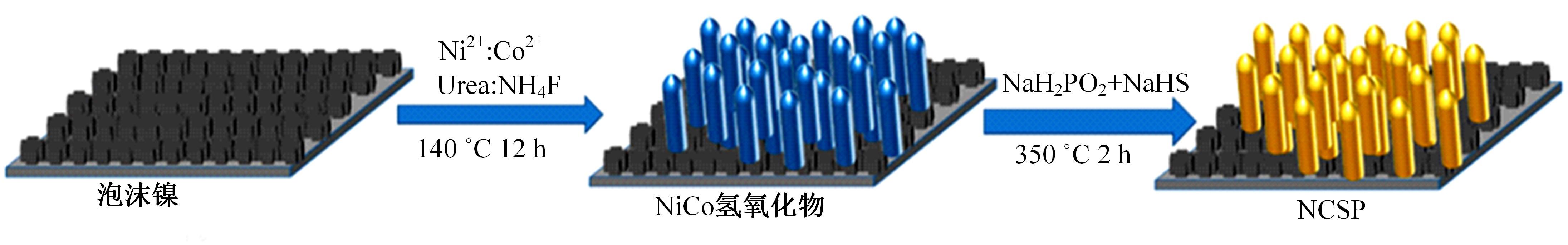
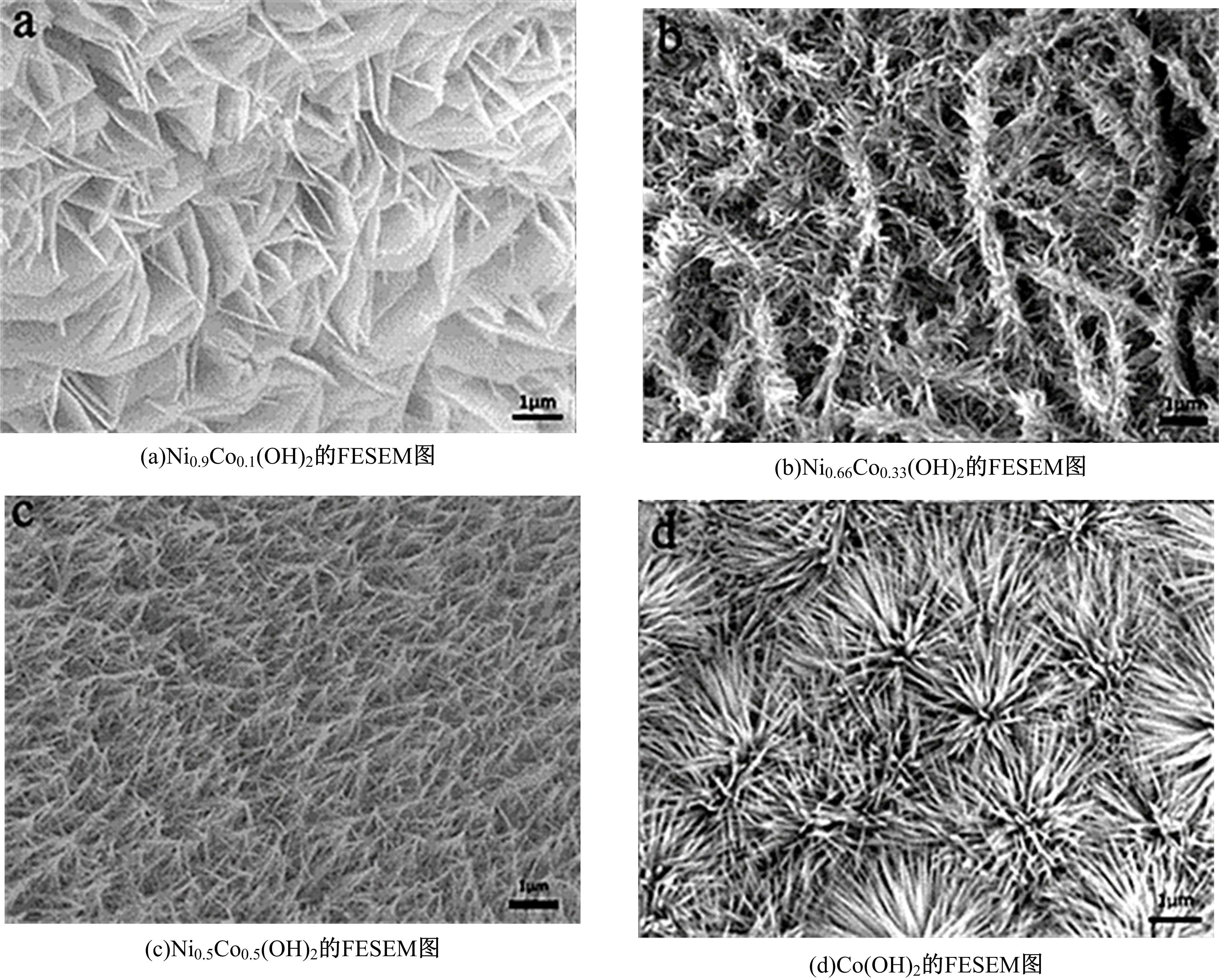
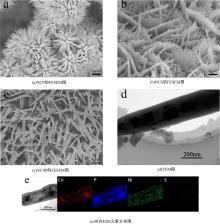
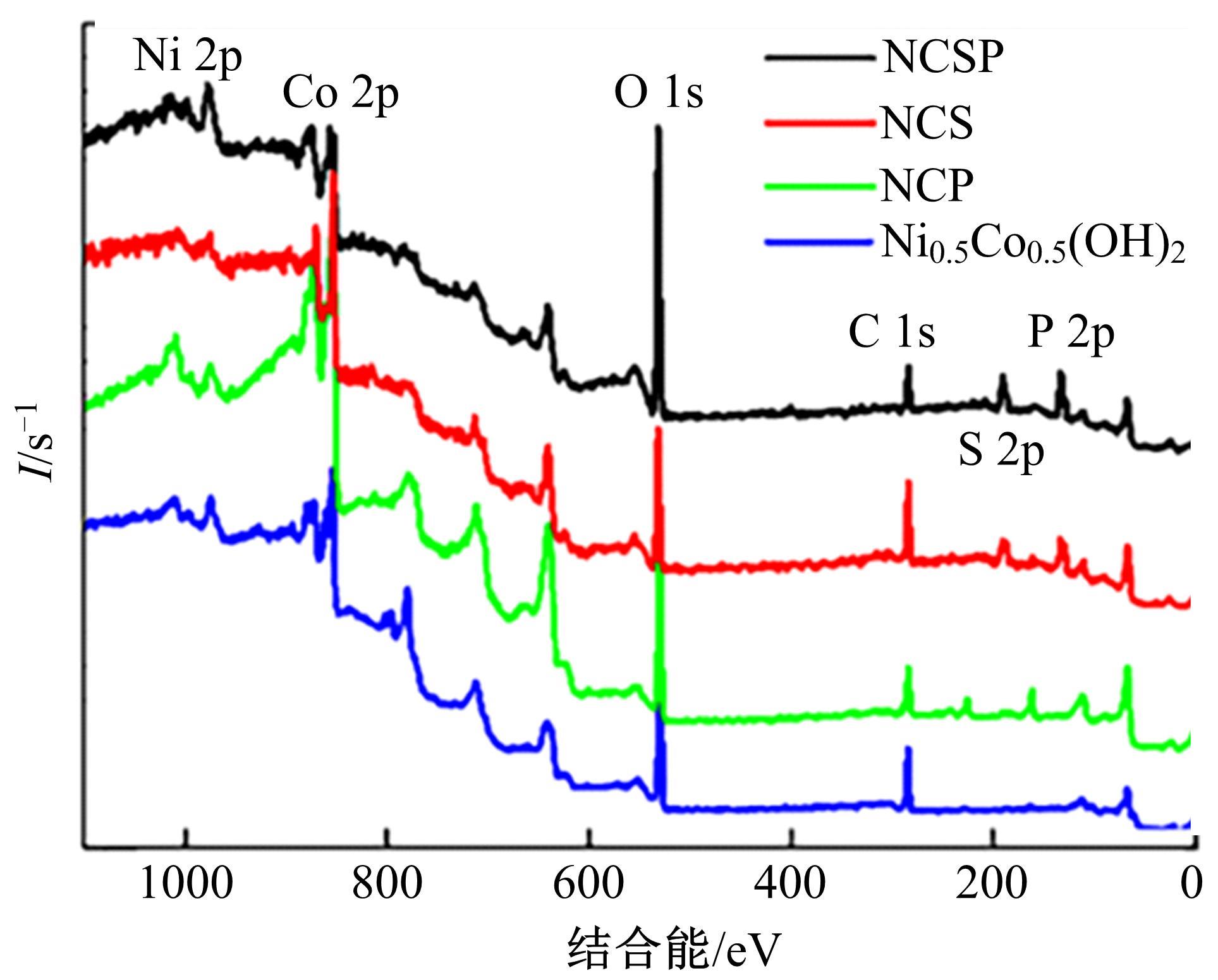
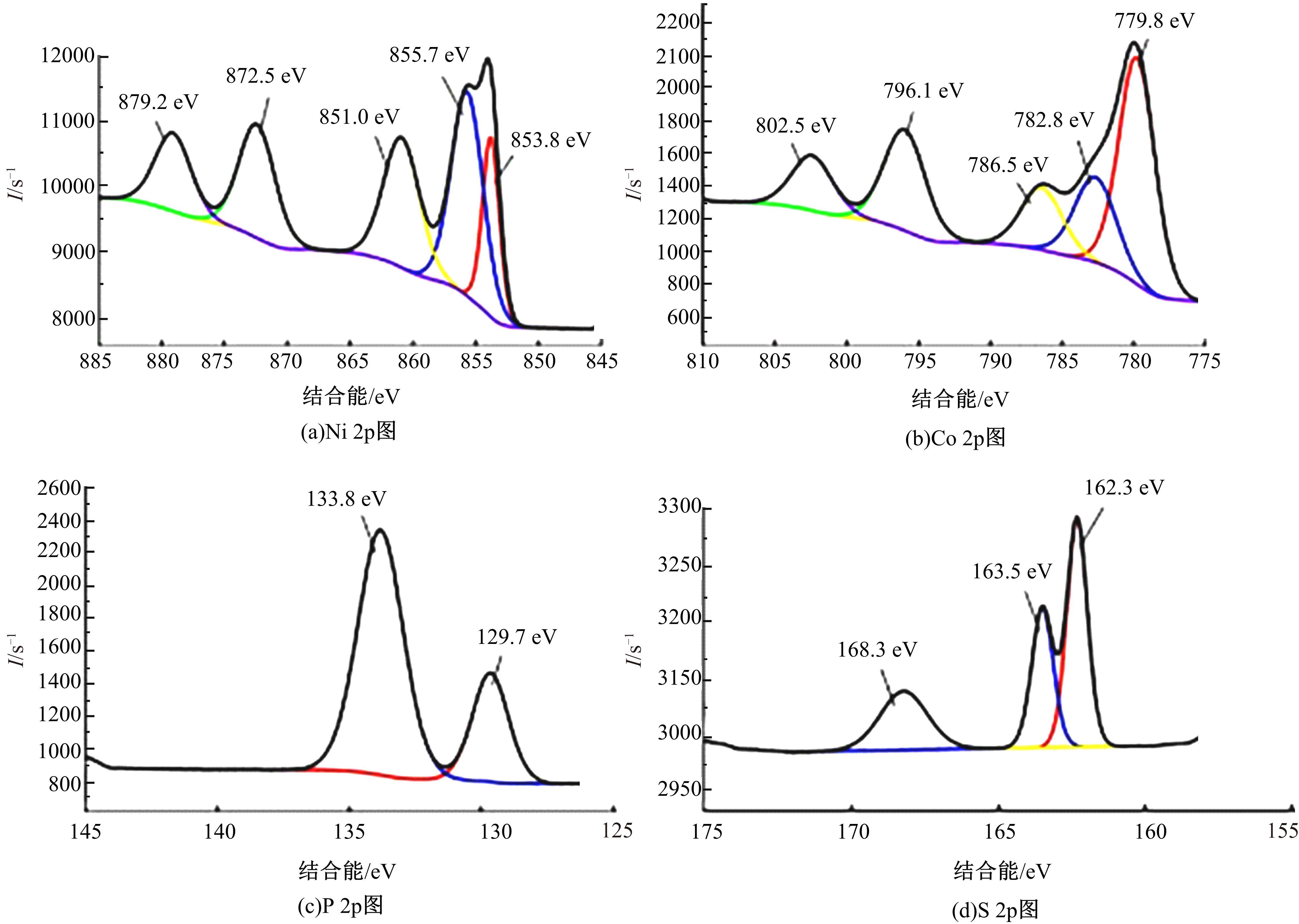
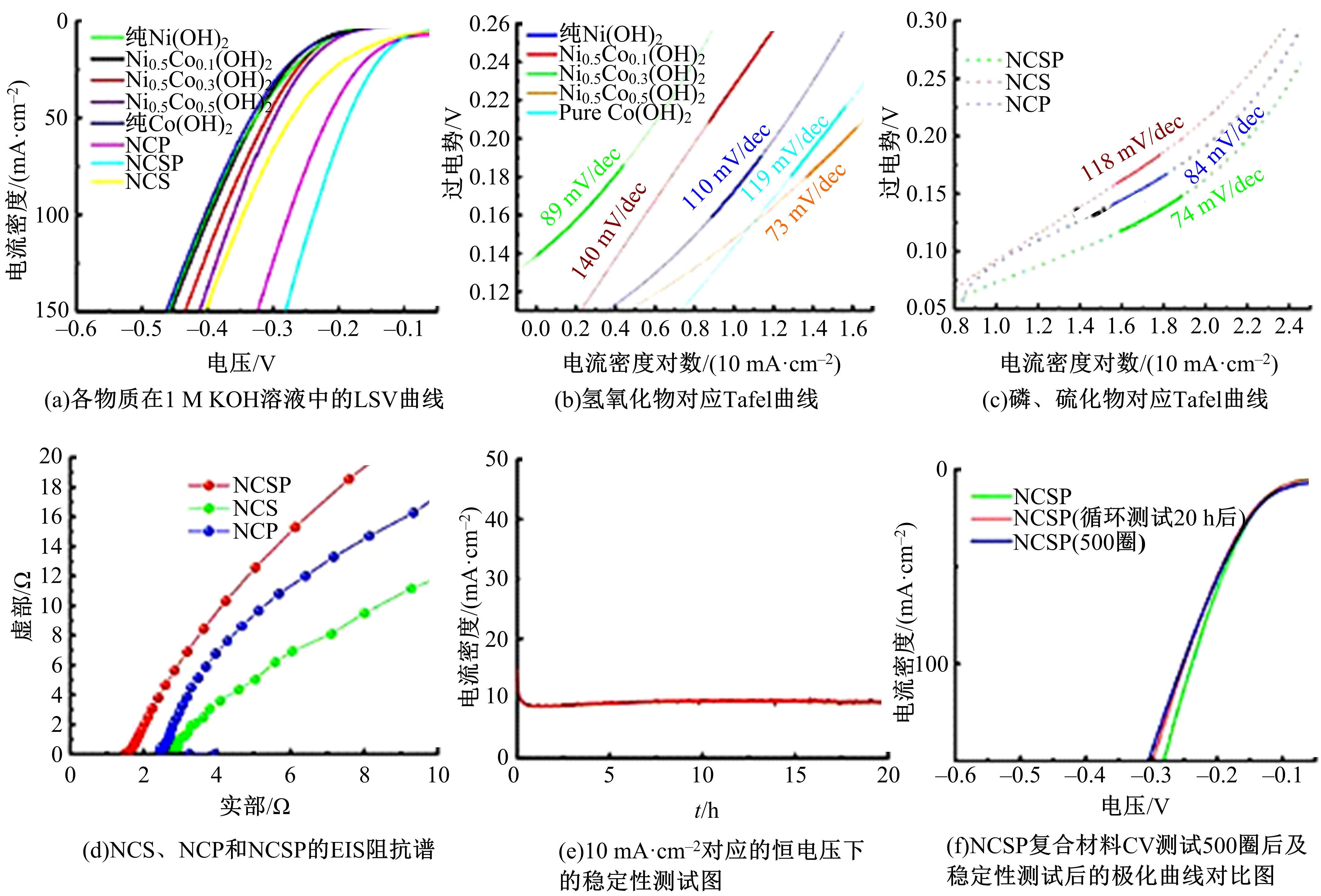
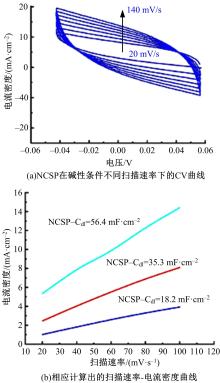
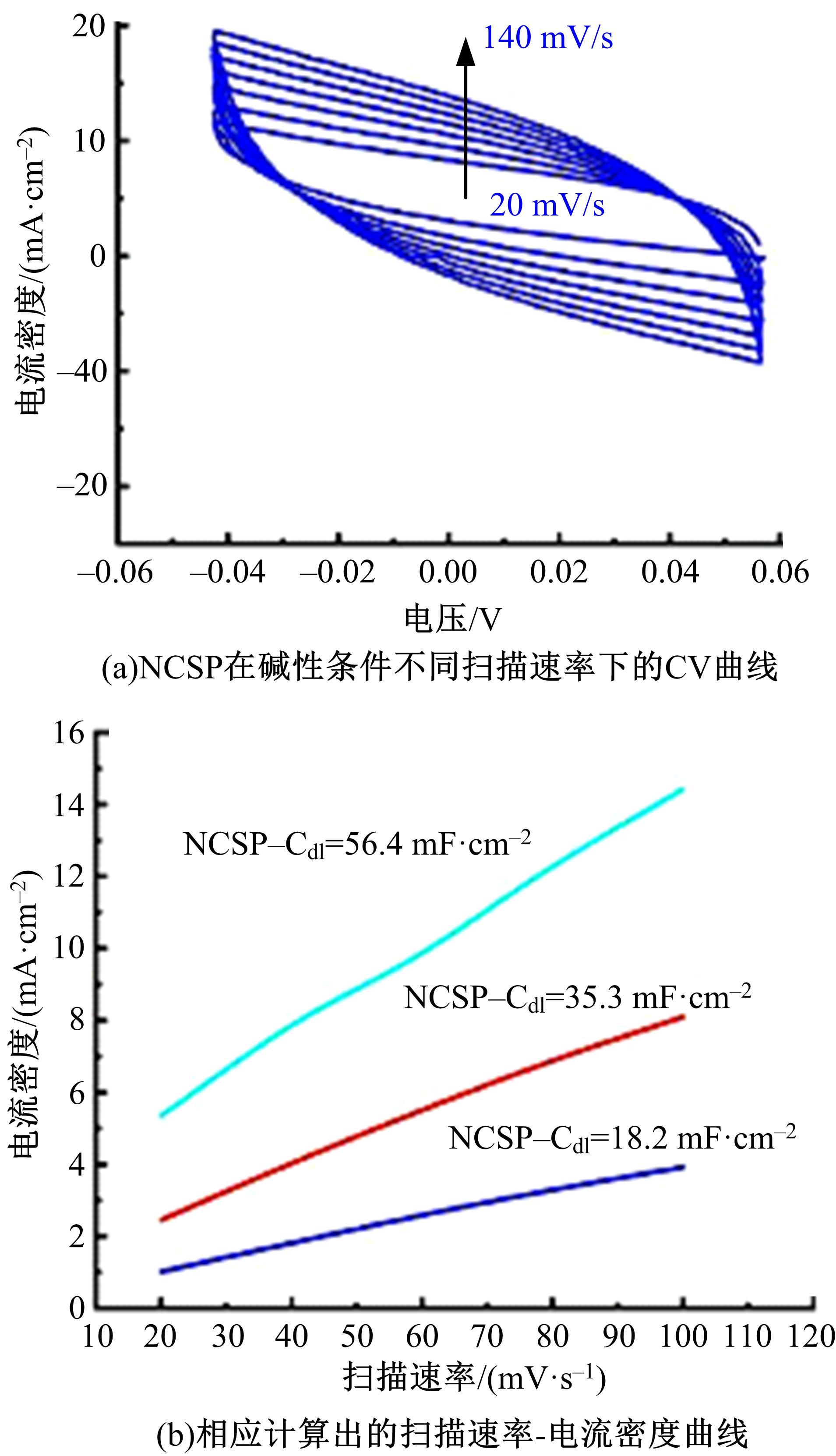
为寻求廉价、性能优异且反应稳定的碱性析氢催化剂以代替贵金属,以过渡金属Ni、Co为研究对象,通过一步煅烧处理得到Ni-Co硫/磷化物催化剂,借助X射线检测和能谱分析等方法验证了催化剂的成功制备。电化学测试结果表明,由于阴/阳离子之间的协同作用,所制备的Ni-Co硫/磷化物的催化活性和电荷转移速率均超过Ni-Co氢氧化物/硫化物/磷化物。Ni-Co硫/磷化物仅需要106 mV的过电势就可以达到10 mA·cm-2的电流密度,循环寿命超过20 h。
中图分类号:
- TB332
| 1 | Cao S, Wang C J, Fu W F, et al. Metal phosphides as Co-Catalysts for photocatalytic and photoelectrocatalytic water splitting[J]. ChemSusChem, 2017, 10(22): 4306-4323. |
| 2 | Guo L, Zhao Y, Yao Z. Mechanical mixtures of metal oxides and phosphorus pentoxide as novel precursors for the synthesis of transition-metal phosphides[J]. Dalton Trans, 2016, 45(3): 1225-1232. |
| 3 | Hong L F, Guo R T, Yuan Y, et al. Recent progress of transition metal phosphides for photocatalytic hydrogen evolution[J]. ChemSusChem, 2020, 14(2): 539-557. |
| 4 | Li R, Zang J, Li W, et al. Three-dimensional transition metal phosphide heteronanorods for efficient overall water splitting[J]. ChemSusChem, 2020, 13(14): 3718-3725. |
| 5 | 王凤武, 朱传高, 方文彦,等. 有机体系中制备纳米TiO2掺杂Ni电极及其性能测试[J]. 吉林大学学报:工学版, 2006(6): 861-865. |
| Wang Feng-wu, Zhu Chuan-guo, Fang Wen-yan, et al. Preparation of TiO2 nanodoped Ni electrodes in organic systems and their performance tests[J]. Journal of Jilin University (Engineering Edition), 2006(6): 861-865. | |
| 6 | Yin J, Wu B, Wang Y, et al. Novel elastic, lattice dynamics and thermodynamic properties of metallic single-layer transition metal phosphides: 2H-M 2P (Mo2P, W2P, Nb2P and Ta2P)[J]. Journal of Physics: Condensed Matter, 2018, 30(13): No. 135701. |
| 7 | Su P, Li Y, Zhang J, et al. Characterization and chemical fixation of stainless steel pickling residue using sodium sulfide hydrate[J]. Environmental Science and Pollution Research, 2019, 26(10): 10240-10250. |
| 8 | Kong D S, Cha J J, Wang H, et al. First-row transition metal dichalcogenide catalysts for hydrogen evolution reaction[J]. Energy & Environmental Science, 2013, 6(12): 3553-3558. |
| 9 | Peng S, Li L, Han X, et al. Cobalt sulfide nanosheet/graphene/carbon nanotube nanocomposites as flexible electrodes for hydrogen evolution[J]. Angewandte Chemie, 2014, 126(46): 12802-12807. |
| 10 | Pan Y, Chen Y, Lin Y, et al. Cobalt nickel phosphide nanoparticles decorated carbon nanotubes as advanced hybrid catalysts for hydrogen evolution[J]. Journal of Materials Chemistry A, 2016, 4: 14675-14686. |
| 11 | Xin Y, Kan X, Gan L, et al. Heterogeneous Bimetallic Phosphide/Sulfide Nanocomposite for Efficient Solar-Energy-Driven Overall Water Splitting[J]. ACS Nano, 2017, 11: 10303-10312. |
| 12 | Acevedo M, Stone M, Schmidt J, et al. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide[J]. Nature Materials, 2015, 14: 1245-1251. |
| 13 | Liu W, Hu E, Jiang H, et al. A highly active and stable hydrogen evolution catalyst based on pyrite-structured cobalt phosphosulfide[J]. Nature Communications, 2016, 7: No. 10771. |
| 14 | Xiao J, Zeng X, Chen W, et al. High electrocatalytic activity of self-standing hollow NiCo2S4 single crystalline nanorod arrays towards sulfide redox shuttles in quantum dot-sensitized solar cells[J]. Chemical Communications, 2013, 49: 11734-11736. |
| 15 | Yan X, Tian L, Chen X. Crystalline/amorphous Ni/NiO core/shell nanosheets as highly active electrocatalysts for hydrogen evolution reaction[J]. Journal of Power Sources, 2015, 300: 336-343. |
| 16 | Feng Y, Yu X, Paik U. Nickel cobalt phosphides quasi-hollow nanocubes as an efficient electrocatalyst for hydrogen evolution in alkaline solution[J]. Chemical Communications, 2016, 52: 1633-1636. |
| 17 | Gong Y Q, Xu Z F, Pan H L, et al. A 3D well-matched electrode pair of Ni-Co-S//Ni-Co-P nanoarrays grown on nickel foam as a high-performance electrocatalyst for water splitting[J]. Journal of Materials Chemistry A, 2018, 6: 12506-12514. |
| 18 | Kim J, Li X, Kang B, et al. High-rate performance of a mixed olivine cathode with off-stoichiometric composition[J]. Chemical Communications, 2015, 51: 13279-13282. |
| 19 | Xin Y, Kan X, Gan L Y, et al. Heterogeneous bimetallic phosphide/sulfide nanocomposite for efficient solar-energy-driven overall water splitting[J]. ACS nano, 2017, 11(10): 10303-10312. |
| 20 | Li Y, Jia B, Chen B, et al. MOF-derived Mn doped porous CoP nanosheets as efficient and stable bifunctional electrocatalysts for water splitting[J]. Dalton Transactions, 2018, 47(41): 14679-14685. |
| 21 | Feng Y, Yu X Y, Paik U. Nickel cobalt phosphides quasi-hollow nanocubes as an efficient electrocatalyst for hydrogen evolution in alkaline solution[J]. Chemical Communications, 2016, 52(8): 1633-1636. |
| 22 | Huang Z, Liu J, Xiao Z, et al. A MOF-derived coral-like NiSe@ NC nanohybrid: an efficient electrocatalyst for the hydrogen evolution reaction at all pH values[J]. Nanoscale, 2018, 10(48): 22758-22765. |
| 23 | Cheng X, Pan Z, Lei C, et al. A strongly coupled 3D ternary Fe2O3@Ni2P/Ni(PO3)2 hybrid for enhanced electrocatalytic oxygen evolution at ultra-high current densities[J]. Journal of Materials Chemistry A, 2019, 7(3): 965-971. |
| 24 | Xu K, Ding H, Zhang M, et al. Regulating water‐reduction kinetics in cobalt phosphide for enhancing HER catalytic activity in alkaline solution[J]. Advanced Materials, 2017, 29(28): No. 1606980. |
| 25 | Cabán-Acevedo M, Stone M L, Schmidt J R, et al. Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide[J]. Nature Materials, 2015, 14(12): 1245-1251. |
| 26 | 张蕾, 张磊, 舒新前, 等. 核桃壳催化热解制取氢气[J]. 吉林大学学报: 工学版, 2008, 38(2): 287-291. |
| Zhang Lei, Zhang Lei, Shu Xin-qian, et al. Catalytic pyrolysis of walnut shells for hydrogen production[J]. Journal of Jilin University (Engineering and Technology Edition), 2008, 38(2): 287-291. |
| [1] | 许良,肖景厚,宋万万,周松. 碳纤维复合材料层合板三点弯曲疲劳性能[J]. 吉林大学学报(工学版), 2024, 54(2): 400-409. |
| [2] | 许良,边钰博,周松,肖景厚. 高温水浸对T800/环氧树脂基复合材料性能的影响[J]. 吉林大学学报(工学版), 2023, 53(7): 1943-1950. |
| [3] | 谢超,王起才,于本田,李盛,林晓旭,鲁志铭. 聚氨酯涂膜弹性模量的AFM测定及微观结构分析[J]. 吉林大学学报(工学版), 2023, 53(5): 1322-1330. |
| [4] | 魏素凤,平昕,李春霖,王国勇. 氮掺杂Co/Co3O4@C核壳纳米粒子作为锂电池负极材料的性能[J]. 吉林大学学报(工学版), 2023, 53(2): 376-384. |
| [5] | 邓海,王超,杨京浩,王利忠,王明辉,李志刚. 碳纤维增强热塑性复合材料研究进展[J]. 吉林大学学报(工学版), 2023, 53(1): 18-30. |
| [6] | 卫宇璇,张明,刘佳,刘硕,路明雨,王洪雨. 基于模态缺陷的变刚度复合材料圆柱壳屈曲特性[J]. 吉林大学学报(工学版), 2022, 52(1): 91-100. |
| [7] | 张霖, 赵宏伟, 杨倚寒, 马智超, 黄虎, 马志超. 单层石墨烯薄膜材料纳米压痕过程的分子动力学解析[J]. 吉林大学学报(工学版), 2013, 43(06): 1558-1565. |
| [8] | 井琦,张文熊,刘晶冰. 热致聚酰胺液晶增韧尼龙6及共混物的形貌[J]. 吉林大学学报(工学版), 2011, 41(05): 1310-1316. |
| [9] | 代汉达, 曲建俊, 庄乾兴. 模压工艺对CF+G/PEEK复合材料力学性能的影响[J]. 吉林大学学报(工学版), 2010, 40(02): 457-0460. |
| [10] | 井琦, 张文熊, 刘晶冰. 热致聚酰胺液晶与尼龙6复合材料的相容性[J]. 吉林大学学报(工学版), 2010, 40(02): 452-0456. |
| [11] | 井琦,张文熊,刘晶冰. 不添加相容剂的尼龙6 /热致液晶聚酰胺复合材料的热性能与结晶行为[J]. 吉林大学学报(工学版), 2009, 39(增刊2): 287-0291. |
| [12] | 刘秀奇,,邢贺钦3,张国,,王立艳4,金晶, . 废弃矿渣粉填充EPDM泡沫型复合材料的制备及其吸油特性[J]. 吉林大学学报(工学版), 2009, 39(01): 56-60. |
| [13] | 李红姬,赫然,张万喜,孙国恩,张莉,牛永盛 . 纳米TiO2/EVA共混复合材料的制备及其性能[J]. 吉林大学学报(工学版), 2006, 36(05): 710-0714. |
| [14] | 李朝辉,,连建设,李光玉. 柠檬酸与金属离子的摩尔比对溶胶凝胶法合成NiO/Ce0.8Gd0.2O1.9复合纳米粉的影响[J]. 吉林大学学报(工学版), 2006, 36(增刊1): 79-0083. |
| [15] | 孙国恩, 张莉, 李红姬, 张春玲, 梁继才, 张万喜. 纳米复合材料的结构与性能[J]. 吉林大学学报(工学版), 2005, 35(06): 577-0581. |
|
||