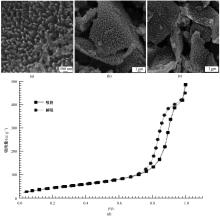
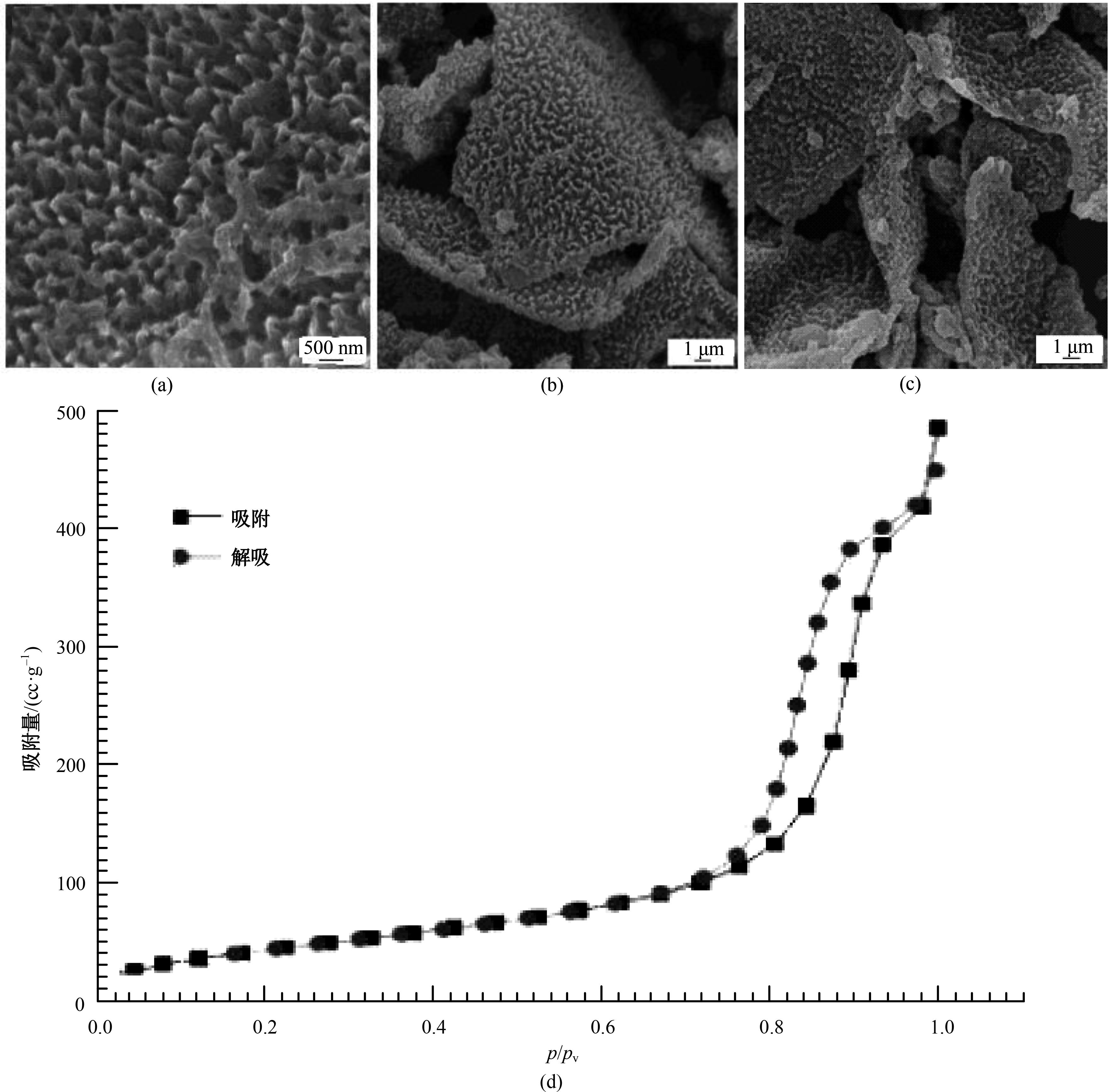
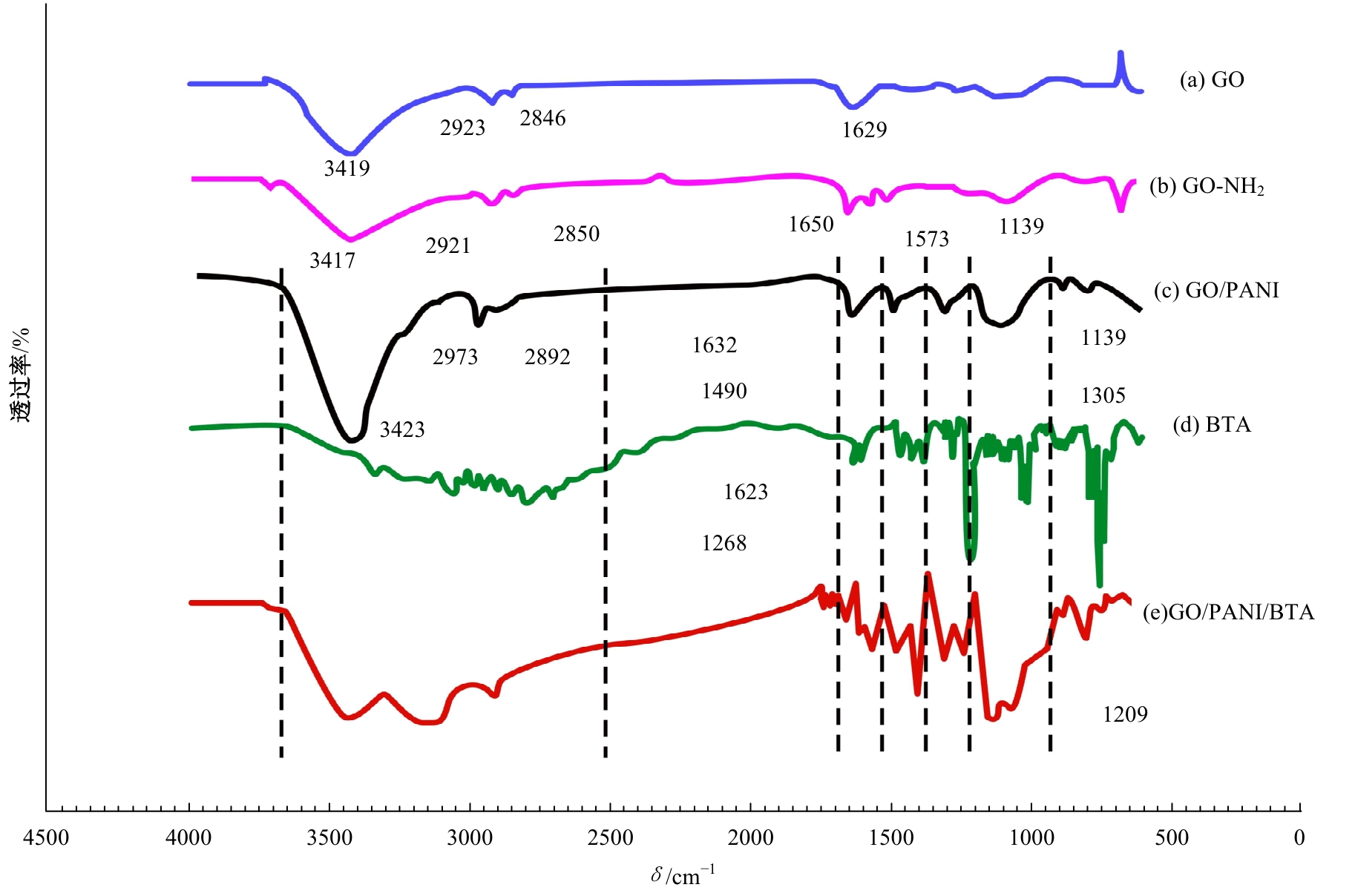
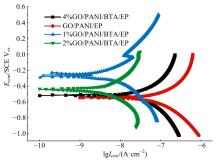
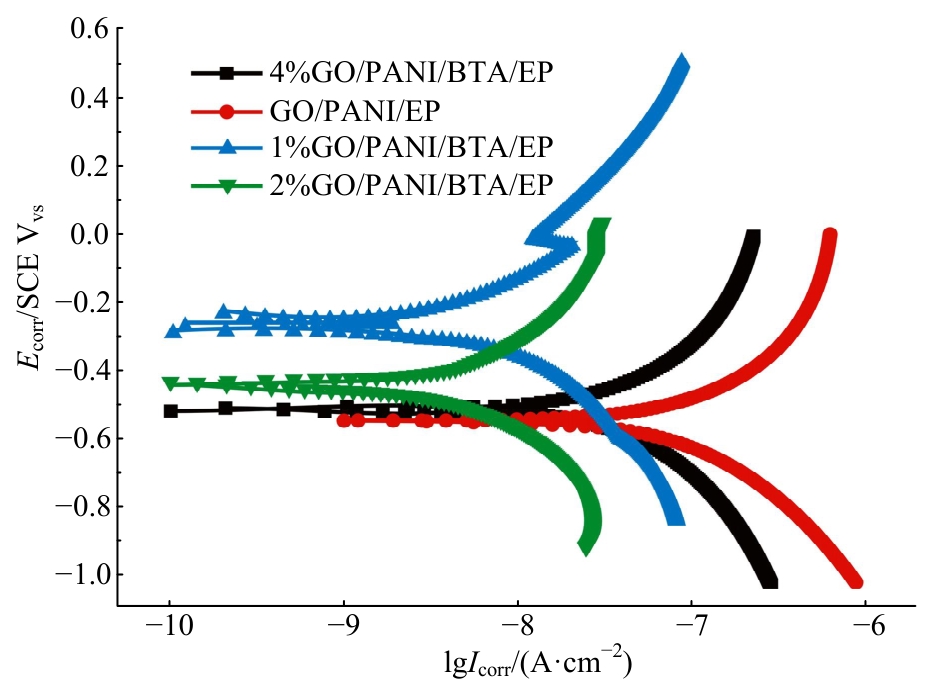
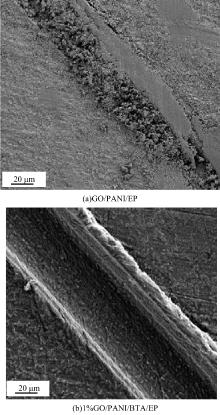
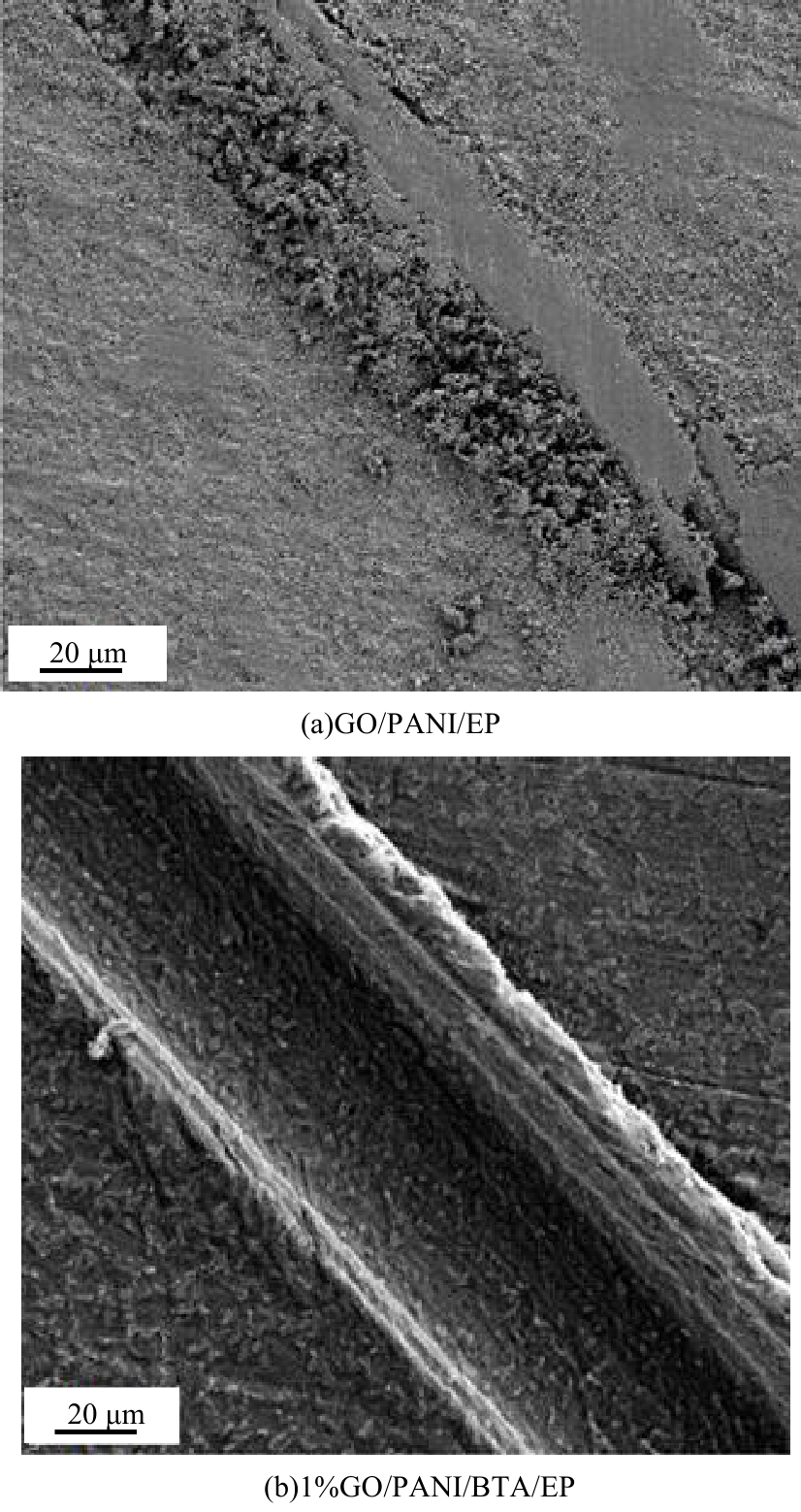
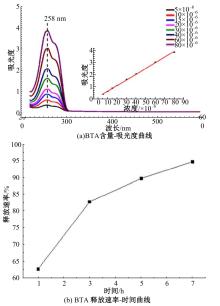
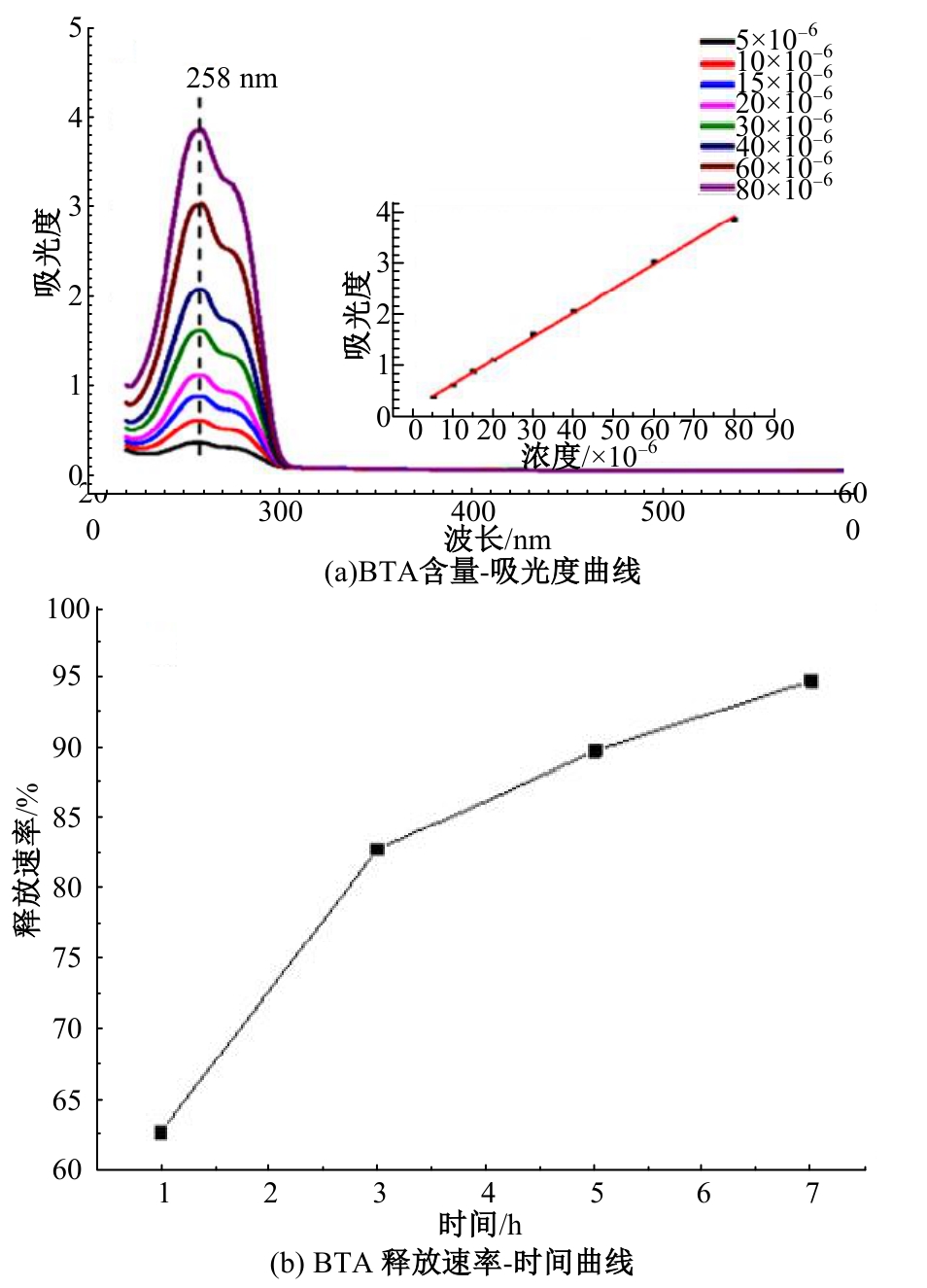
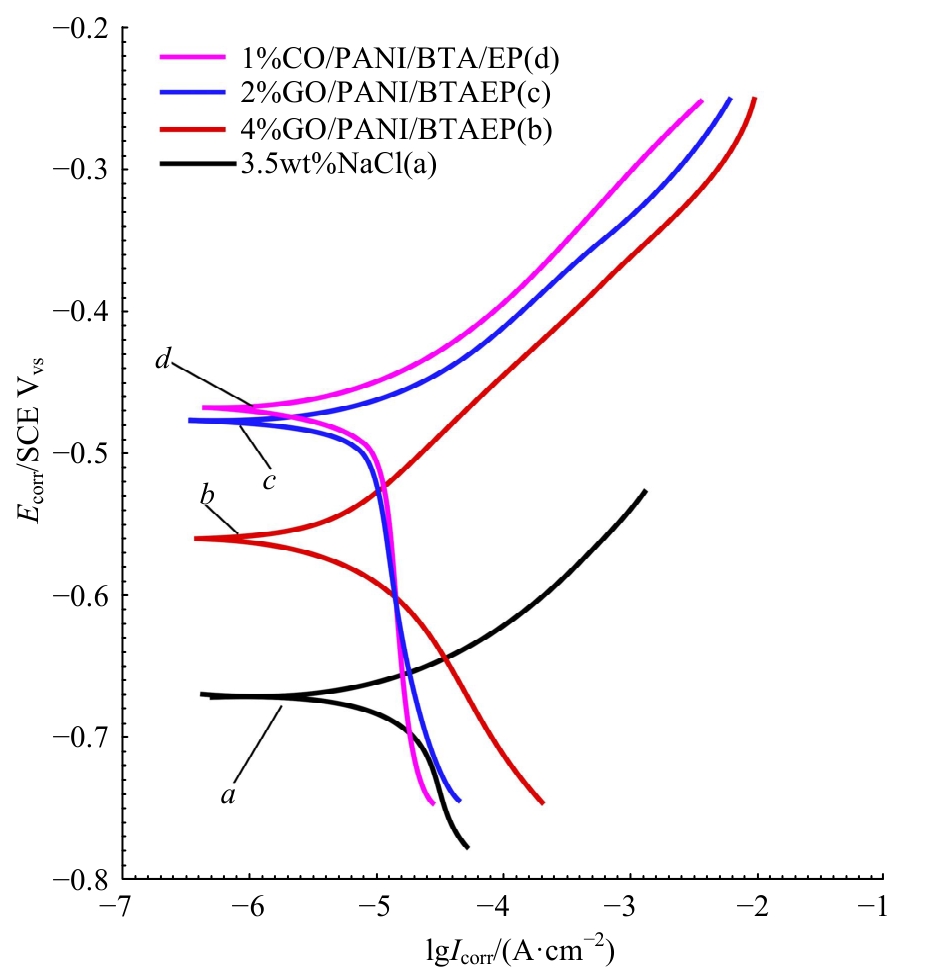
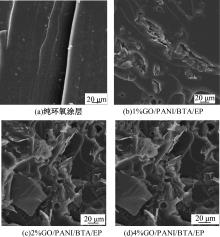
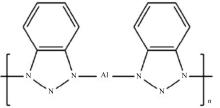
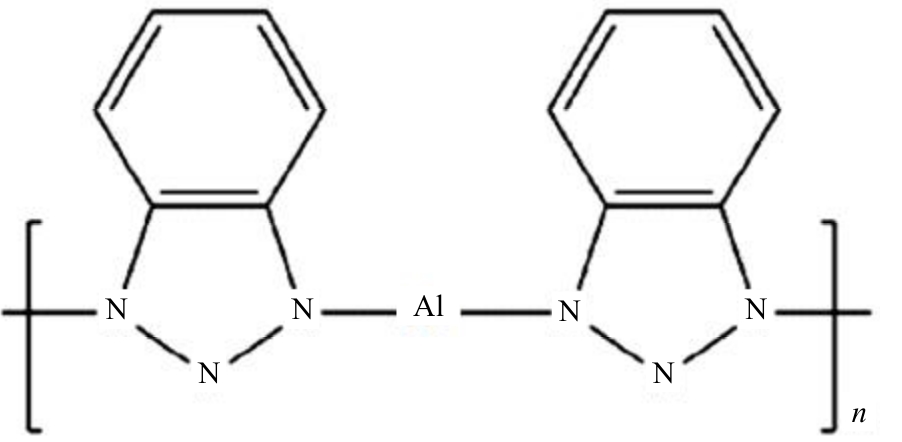
| [1] |
Huang G L, Xue M L, Zi Y Z. Review on the corrosion and protection mechanism of metal materials[J]. World Nonferrous Metals, 2018(6): 217-218.
|
| [2] |
Zhang D Q, Gao L X, Zhou G D. Research, development and prospect of corrosion inhibitors athome and abroad[J]. Corrosion and Protection, 2009, 30: 604-610.
|
| [3] |
Zou X, Xiang Q, Hao J, et al. Scalable modulation of reduced graphene oxide properties via regulatinggraphite oxide precursors[J]. Journal of Alloys and Compounds, 2019, 791: 423-430.
|
| [4] |
黎晓琳, 孔纲, 车淳山, 等. 改性氧化石墨烯在金属防腐蚀涂层中的研究进展[J]. 电镀与涂饰, 2021, 40(12): 929-936.
|
|
Li Xiao-lin, Kong Gang, Che Chun-shan,et al. Research progress of modified graphene oxide in metal anti-corrosion coatings[J]. Electroplating and Coating, 2021, 40(12): 929-936.
|
| [5] |
Mohammadkhani R, Ramezanzadeh M, Saadatmandi S, et al. Designing a dual-functional epoxy composite system with self-healing/barrier anti-corrosion performance using graphene oxide nano-scale platforms decorated with zinc doped-conductive polypyrrole nanoparticles with great environmental stability and non-toxicity[J].Chemical Engineering Journal, 2020,382: 122819.
|
| [6] |
Hao Y S, Zhao Y F, Li B, et al. Self-healing effect of graphene@PANI loaded with benzotriazole for carbon steel [J]. Corrosion Science, 2020, 163: 108246.
|
| [7] |
Kasaeian M, Ghasemi E, Ramezanzadeh B, et al. Construction of a highly effective self-repair corrosion-resistant epoxy composite through impregnation of 1H-Benzimidazole corrosion inhibitor modified graphene oxide nanosheets (GO-BIM)[J]. Corrosion Science, 2018, 145: 119-134.
|
| [8] |
Garcia H M, Jimenez M A, Casal B, et al. Preparation and electrochemical study of cerium-silica sol-gel thin films[J]. Journal of Alloys and Compounds, 2004, 380(1/2): 219-224.
|
| [9] |
Shchukin D G, Möhwald H. Self-repairing coatings containing active nanoreservoirs[J]. Small, 2007, 3(6): 926-943.
|
| [10] |
Zhou Y, Zuo Y, Lin B. The compounded inhibition of sodium molybdate and benzotriazole on pitting corrosion of Q235 steel in NaCl+NaHCO3 solution[J]. Materials Chemistry and Physics, 2017, 192: 86-93.
|
| [11] |
Huang Y, Liu T, Ma L, et al. Saline-responsive triple-action self-healing coating for intelligent corrosion control[J]. Materials & Design, 2022, 214: 110381.
|
| [12] |
赵一帆. 聚苯胺纳米自修复涂层的制备及防腐机理研究[D]. 沈阳: 沈阳化工大学材料科学与工程学院, 2019.
|
|
Zhao Yi-fan. Preparation and anti-corrosion mechanism of polyaniline nano self-healing coatings[D]. Shenyang: School of Materials Science and Engineering, Shenyang University of Chemical Technology, 2019
|
| [13] |
闭锦叶. 负载苯并三氮唑的聚苯胺/环氧涂层的制备及防腐性能研究[D]. 广州: 华南理工大学机械与汽车工程学院, 2022.
|
|
Bi Jin-ye. Preparation and anti-corrosion performance of polyaniline/epoxy coatings loaded with benzotriazole[D]. Guangzhou: School of Machanical & Automotive Engineering, South China University of Technology, 2022
|
| [14] |
Cai K, Zuo S, Luo S, et al. Preparation of polyaniline/graphene composites with excellent anti-corrosionproperties and their application in waterborne polyurethane anticorrosive coatings[J]. RSC Advances, 2016(98): 95965-95972.
|
| [15] |
Chang C H, Huang T C, Peng C W, et al. Novel anticorrosion coatings prepared frompolyaniline/graphene composites[J]. Carbon, 2012, 50(14): 5044-5051.
|
| [16] |
Jafari Y, Ghoreishi S M, Shabani N M. Polyaniline/graphene nanocomposite coatings oncopper: electropolymerization, characterization, and evaluation of corrosion protection performance[J]. Synthetic Metals, 2016, 217: 220-230.
|
| [17] |
Sheng X, Cai W, Li Z, et al. Synthesis of functionalized graphene/polyaniline nanocomposites witheffective synergistic reinforcement on anticorrosion[J]. Industrial & Engineering Chemistry Research, 2016, 55(31): 8576-8585.
|
| [18] |
李红玲. KH-151硅烷改性纳米ZrO2对铝合金表面环氧树脂涂层防护性能的影响[J]. 腐蚀与防护,2023, 44(9): 83-89.
|
|
Li Hong-ling. The effect of KH-151 silane modified nano ZrO2 on the protective performance of epoxy resin coatings on aluminum alloy surfaces [J]. Corrosion and Protection, 2023, 44(9): 83-89.
|
| [19] |
Liu X, Zhang D, Hou P, et al. Preparation and characterization of polyelectrolyte-modified attapulgite as nanocontainers for protection of carbon steel[J]. Journal of the Electrochemical Society, 2018, 165(13): 907-915.
|
| [20] |
Gao Z, Feng W, Chang J, et al. Chemically grafted graphene-polyaniline composite for application in supercapacitor[J]. Electrochimica Acta, 2014, 133(7): 325-334.
|
| [21] |
Chatterjee S, Layek R K, Nandi A K. Changing the morphology of polyaniline from a nanotube to a flat rectangular nanopipe by polymerizing in the presence of amino-functionalized reduced graphene oxide and its resulting increase in photocurrent[J]. Carbon, 2013, 52: 509-519.
|
| [22] |
Kotal M, Bhowmick A K. Multifunctional hybrid materials based on carbon nanotube chemically bonded to reduced graphene oxide[J]. Journal of Physical Chemistry C, 2013, 117(48): 25865-25875.
|
| [23] |
Cruz S R, Romero G J, Angulo S J L, et al. Comparative study of polyaniline cast films prepared from enzymatically and chemically synthesized polyaniline[J]. Polymer, 2004, 45(14): 4711-4717.
|
| [24] |
Poling G W. Reflection infra-red studies of films formed by benzotriazole on Cu[J]. Corrosion Science,1970, 10(5): 359-370.
|
| [25] |
Chen Y, Jiang Y Y, Ye Z Y, et al. Adsorption dynamics of benzotriazole on copper in chloride solution[J]. Corrosion, 2013, 69(9): 886-892.
|
| [26] |
Zhang B, He C, Cheng W, et al. Synergistic corrosion inhibition of environment-friendly inhibitors on the corrosion of carbon steel in soft water[J]. Corrosion Science, 2015, 94(5): 6-20.
|
| [27] |
Okafor P C, Zheng Y. Synergistic inhibition behaviour of methylbenzyl quaternary imidazoline derivative and iodide ions on mild steel in HSO solutions[J]. Corrosion Science, 2009, 51(4): 850-859.
|
| [28] |
Ramezanzadeh B, Niroumandrad S, Ahmadi A, et al. Enhancement of barrier and corrosion protection performance of an epoxy coating through wet transfer of amino functionalized graphene oxide[J]. Corrosion Science, 2016, 103: 283-304.
|
| [29] |
Gupta G, Birbilis N, Cook A B, et al. Polyaniline-lignosulfonate/epoxy coating for corrosion protection of AA2024-T3[J]. Corrosion Science, 2013, 67: 256-267.
|
| [30] |
Fahlman M, Jasty S, Epstein A J. Corrosion protection of iron/steel by emeraldine base polyaniline: an X-ray photoelectron spectroscopy study[J]. Synthetic Metals, 1997, 85(1): 1323-1326.
|
| [31] |
Chen Z, Huang L, Zhang G, et al. Benzotriazole as a volatile corrosion inhibitor during the early stage of copper corrosion under adsorbed thin electrolyte layers[J]. Corrosion Science, 2012, 65: 214-222.
|
| [32] |
Gattinoni C, Michaelides A. Understanding corrosion inhibition with van der Waals DFT methods: the case of benzotriazole[J]. Faraday Discussions, 2015, 180: 439-458.
|