吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (3): 739-748.doi: 10.13481/j.1671-587X.20240318
• 基础研究 • 上一篇
基于幽门螺杆菌hp0169基因三维结构的生物信息学分析
林玲辉1,李娜1,尹晓燕1,王晓凌1,胡亚平1,刘威2,费瑞3( ),田新利1(
),田新利1( )
)
- 1.邢台医学高等专科学校病原生物学与免疫学教研室,河北 邢台 054000
2.陆军军医大学基础医学院全军免疫学研究所,重庆 400038
3.吉林大学基础医学院细胞生物学系,吉林 长春 130021
Bioinformatics anlysis based on three-dimensional structure of Helicobacter pylori hp0169 gene
Linghui LIN1,Na LI1,Xiaoyan YIN1,Xiaoling WANG1,Yaping HU1,Wei LIU2,Rui FEI3( ),Xinli TIAN1(
),Xinli TIAN1( )
)
- 1.Department of Pathogenic Biology and Immunology,Xingtai Medical College,Xingtai 054000,China
2.Institute of Immunology,School of Basic Medical Scienes,Army Medical University,Chongqing 400038,China
3.Department of Cell Biology,School of Basic Medical Scienes,Jilin University,Changchun 130021,China
摘要:
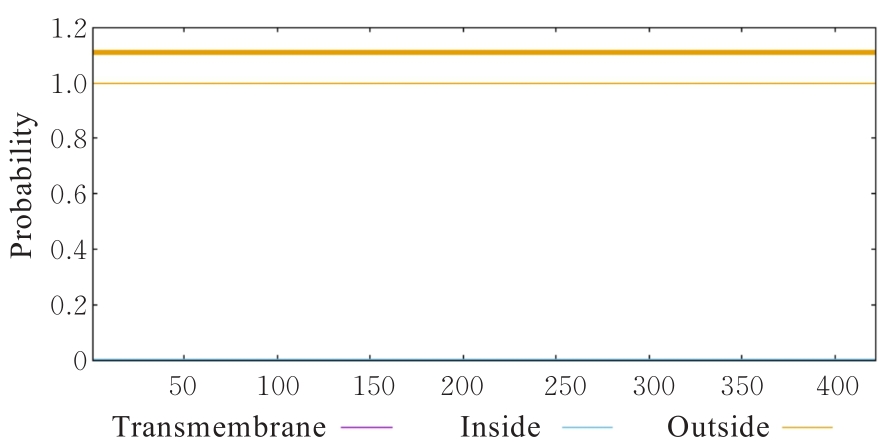
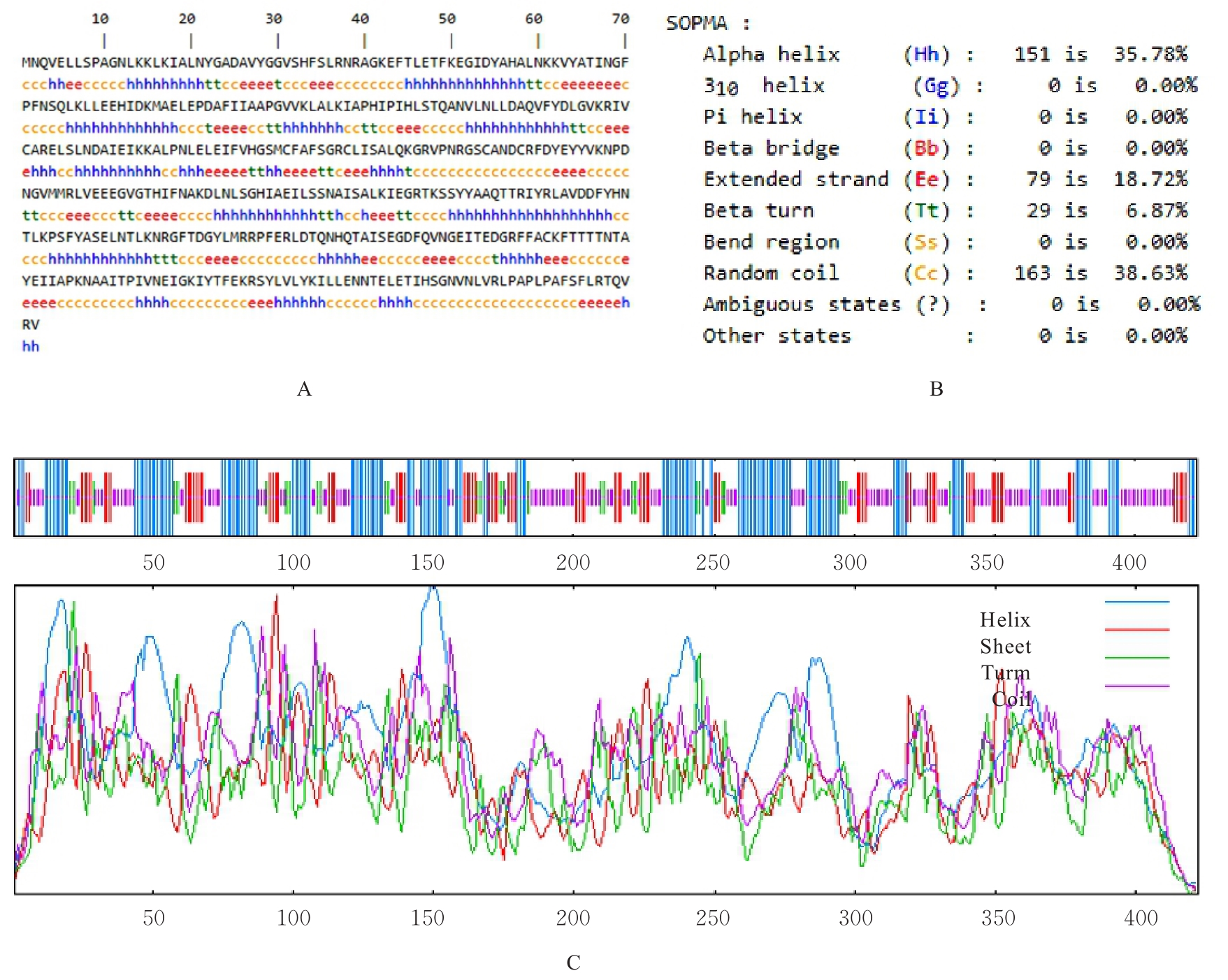
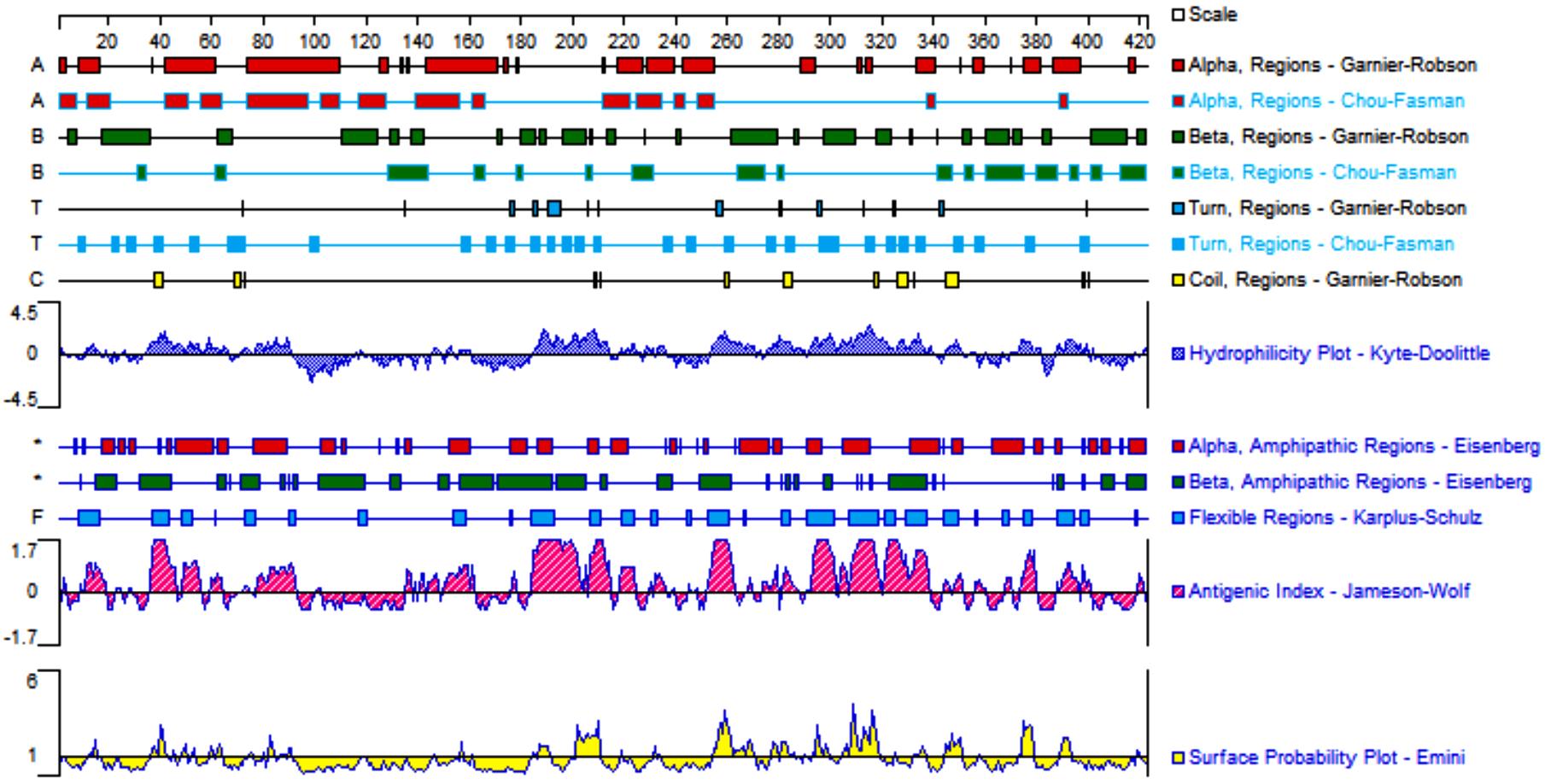
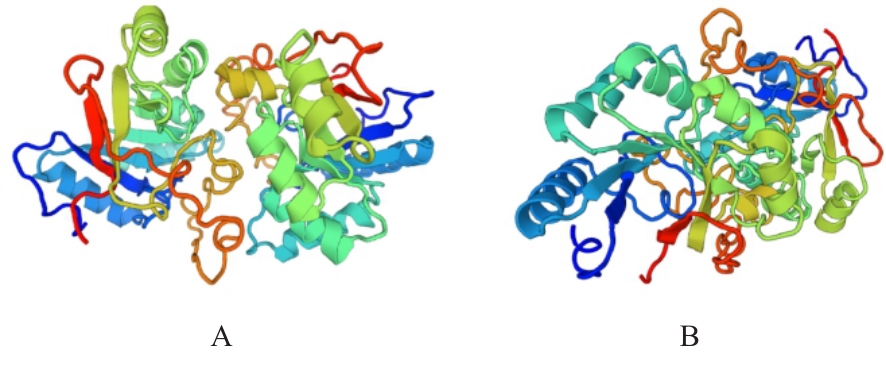
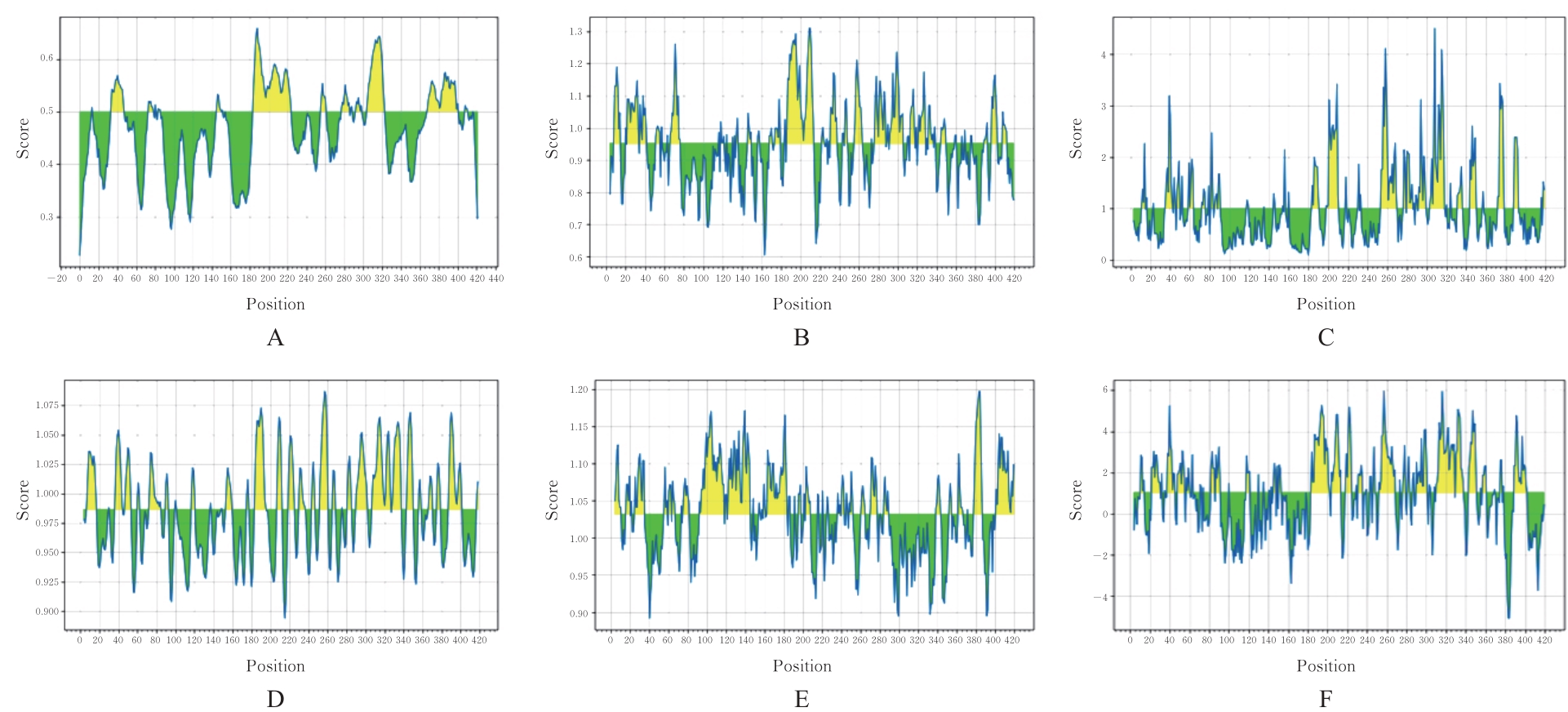
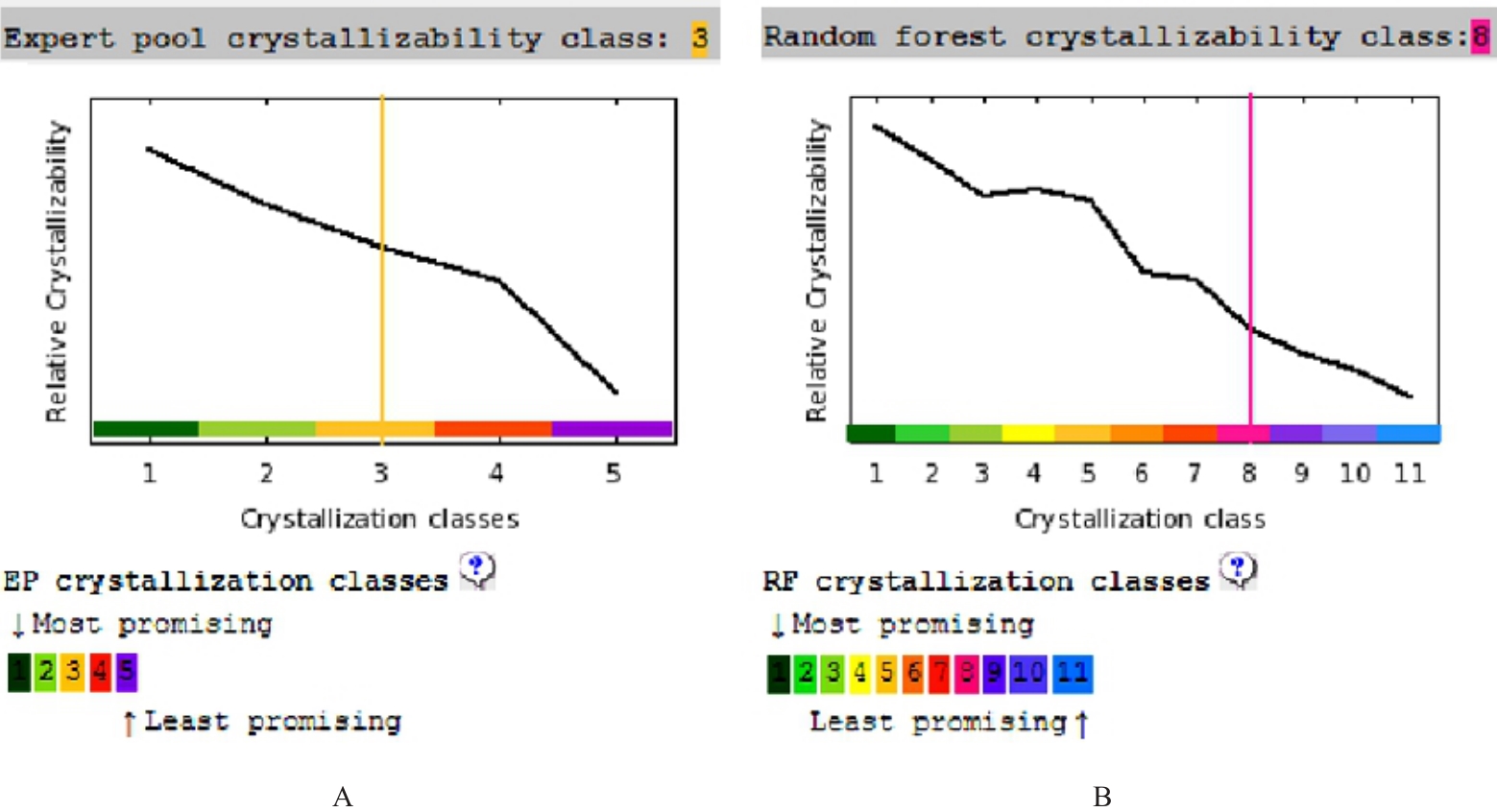
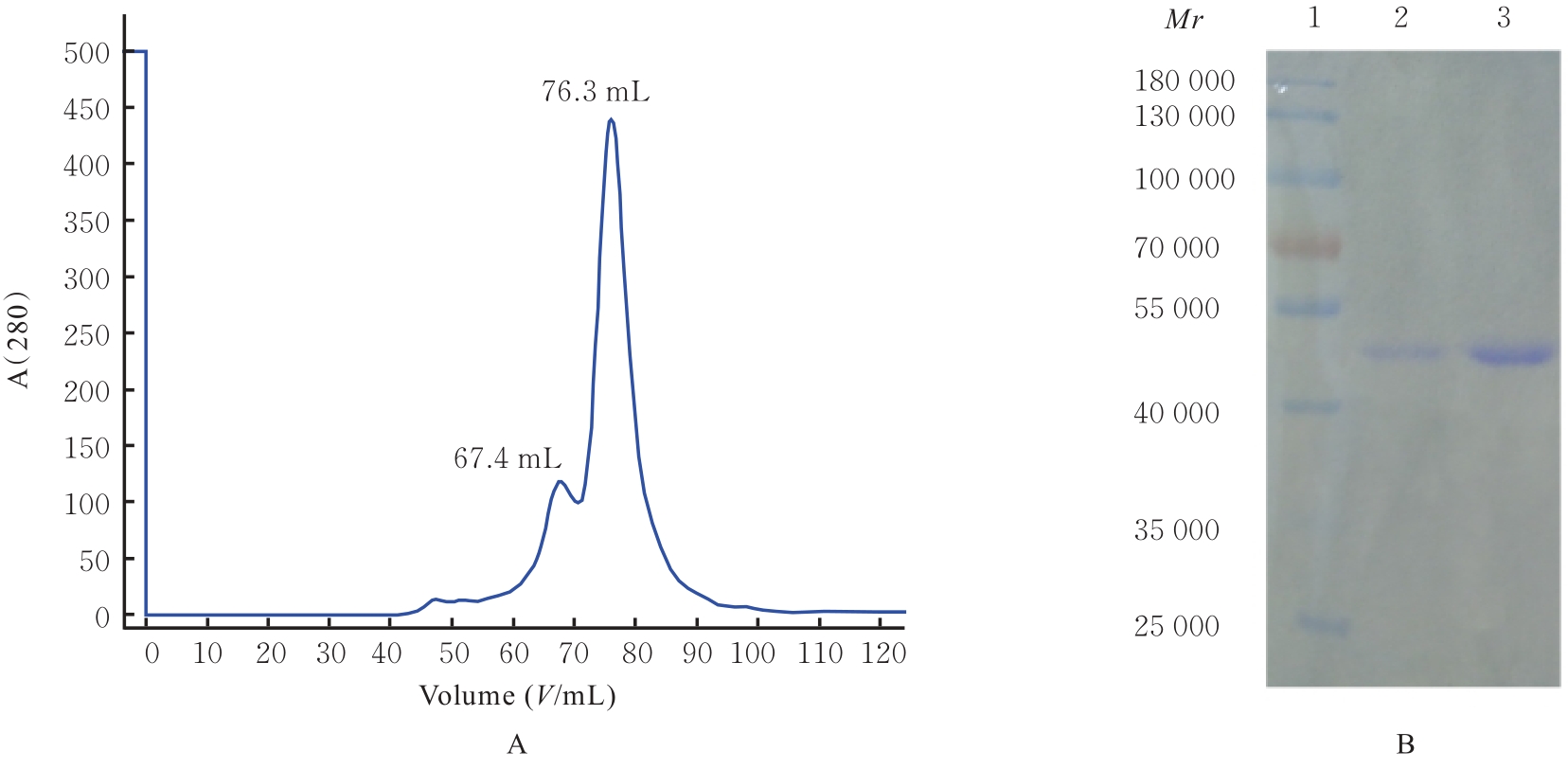
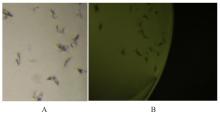
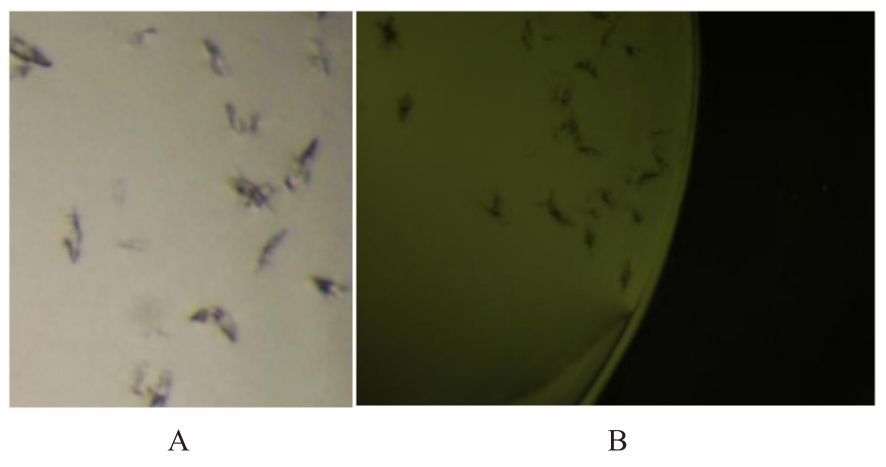
目的 克隆幽门螺杆菌(Hp)hp0169基因并进行晶体学研究,分析其二级结构和三维结构。 方法 由UniProt数据库中检索Hp NCTC26695菌株hp0169基因及其编码蛋白序列,采用生物信息学方法分析Hp重组胶原蛋白酶(HpPrtC)蛋白理化性质,SOPMA和DNAStrar软件预测HpPrtC蛋白二级结构特征,SWISS-MOPEL软件构建HpPrtC蛋白三维结构,IEDB和ABCpred软件预测HpPrtC蛋白B淋巴细胞抗原表位,SYFPEITHI网站预测T淋巴细胞抗原表位,专家库(EP)算法和随机森林(RF)算法预测HpPrtC蛋白可结晶性。原核表达HpPrtC重组蛋白,经Ni2+亲和层析和分子筛技术纯化蛋白,结晶试剂盒筛选HpPrtC的结晶条件。 结果 hp0169基因共包含1 269个碱基配对,编码蛋白全长422个氨基酸,理论等电点为7.64,相对分子质量为47 300。HpPrtC蛋白为亲水性、可溶性蛋白。HpPrtC蛋白α螺旋的氨基酸数量占全部氨基酸数量百分率为35.78%,β片层为18.72%,β转角为6.87%,无规则卷曲为38.63%。抗原表位分析,HpPrtC蛋白含有B淋巴细胞的5个优势线性表位和3个构象表位及多个T淋巴细胞潜在优势抗原表位。同源建模,HpPrtC蛋白呈二聚体,单体由β折叠围成桶状结构,周围被α螺旋和无规则卷曲围绕。HpPrtC蛋白为中等难度结晶,且无信号肽和跨膜螺旋,在0.2 mol·L-1 氯化镁、0.1 mol·L-1 三羟甲基氨基甲烷(Tris)、3.4 mol·L-1 己二醇和pH 8.5条件下出现细小成簇的针状晶体。 结论 HpPrtC是一种亲水型蛋白,呈二聚体构造,在适宜条件下呈细小成簇针状晶体,其具有T淋巴细胞和B淋巴细胞优势抗原表位,可作为Hp疫苗设计的抗原,用于构建多价融合疫苗或多表位疫苗,本研究结果为Hp的防治提供了实验基础。
中图分类号:
- R378.99