吉林大学学报(医学版) ›› 2024, Vol. 50 ›› Issue (6): 1519-1525.doi: 10.13481/j.1671-587X.20240605
• 基础研究 • 上一篇
3D打印PLA/PTMC-Ca₃(PO₄)₂复合支架制备及其对兔骨髓间充质干细胞成骨分化的影响
- 吉林大学第二医院创伤外科,吉林 长春 130022
Preparation of 3D-printed PLA/PTMC-Ca₃(PO₄)₂ composite scaffolds and their effects on osteogenic differentiation of bone marrow mesenchymal stem cells of rabbits
Xingang LIU,Xu CHEN,Yadong LIU,Jinhu MIAO,Guoxi SHAO( )
)
- Department of Trauma Surgery,Second Hospital,Jilin University,Changchun 130022,China
摘要:
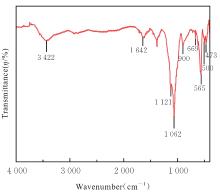
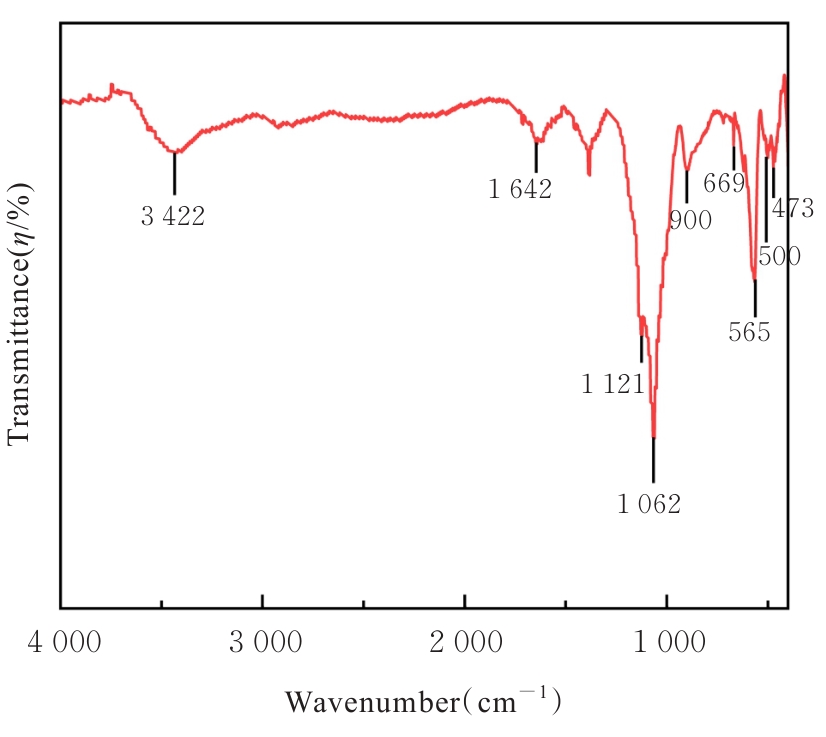
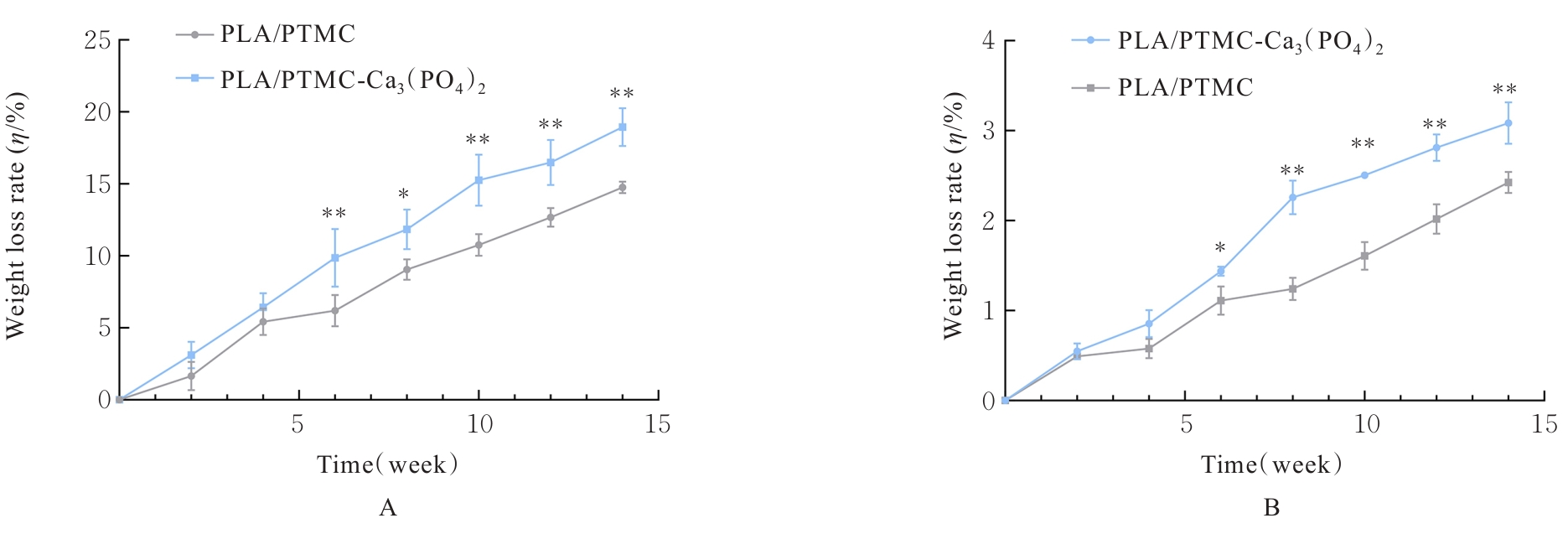
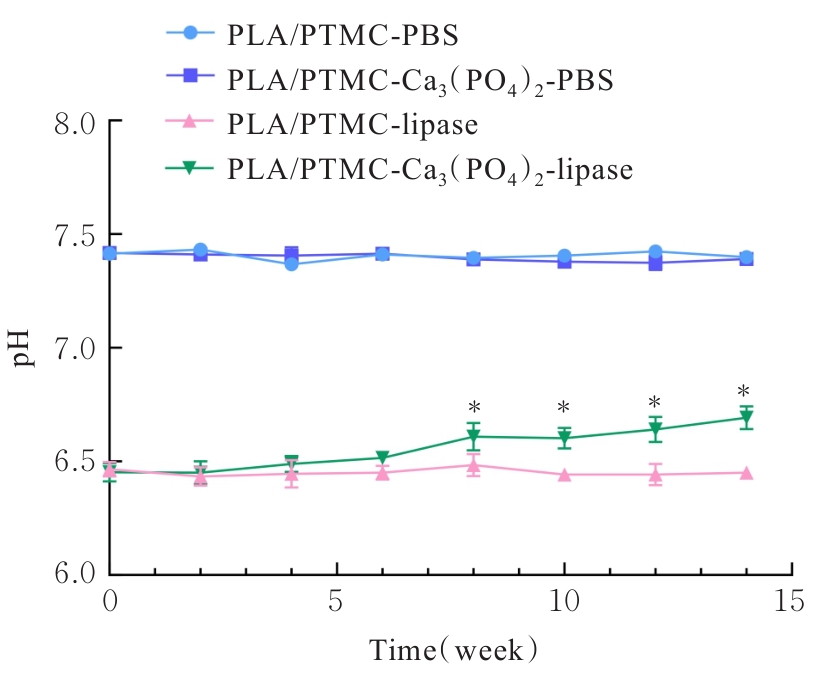
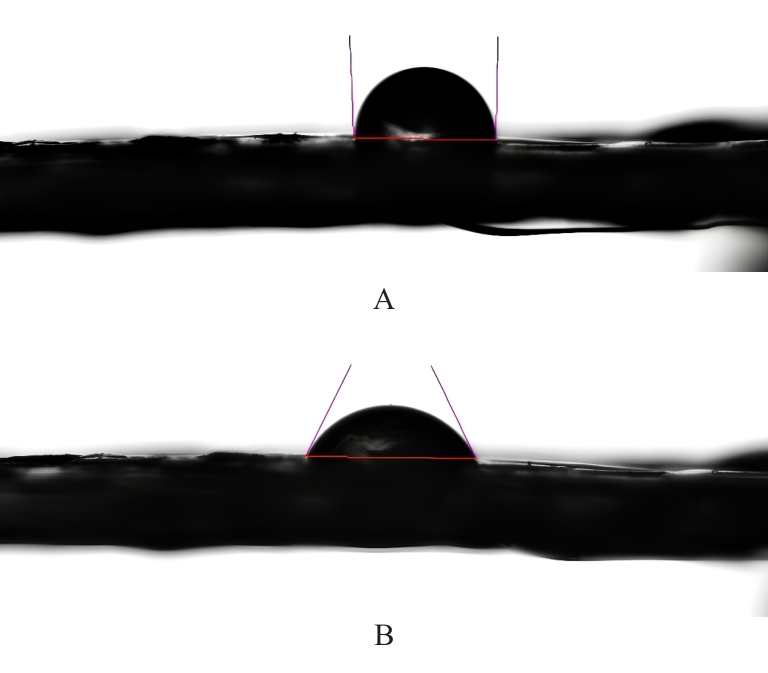
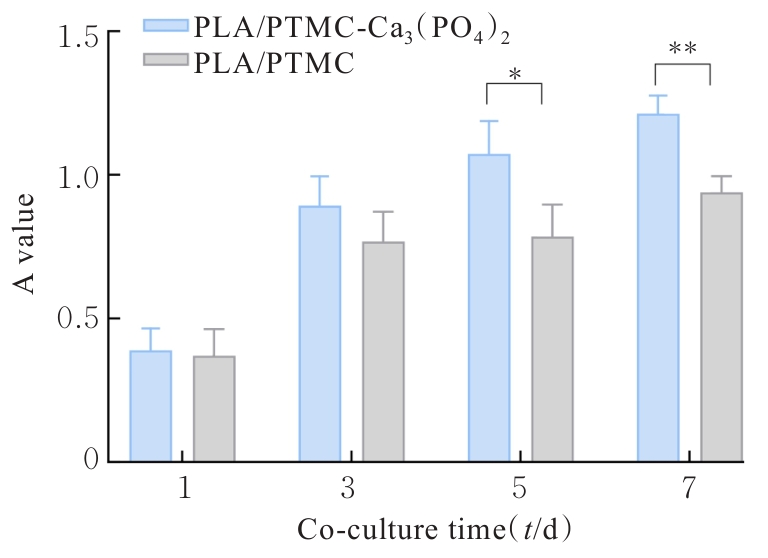
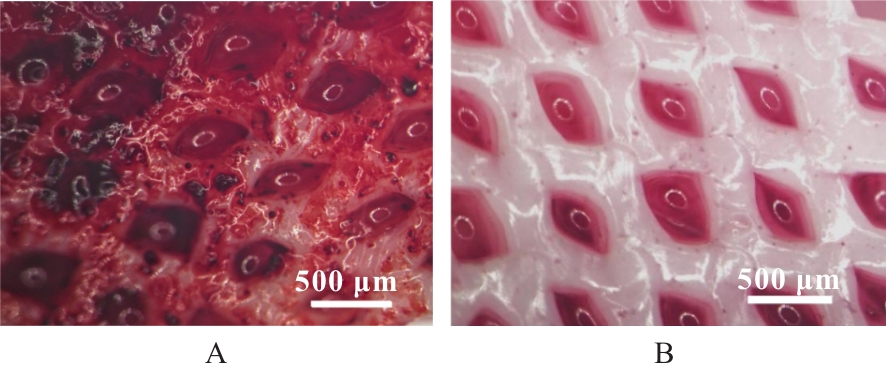
目的 探讨通过3D打印技术制备的聚乳酸(PLA)/聚三亚甲基碳酸酯(PTMC)和PLA/PTMC-磷酸三钙[Ca?(PO?)?]复合多孔支架对兔骨髓间充质干细胞(BMSCs)的影响,阐明其在骨缺损修复中的应用价值。 方法 混合材料后使用桌面挤丝机制备PLA/PTMC和PLA/PTMC-Ca?(PO?)? 线材,使用CATIA V5-6R2019建模软件设计支架,并使用CreatBot F430 3D打印机进行支架制作。红外光谱检测PLA/PTMC-Ca?(PO?)? 支架化学结构,体外降解实验检测2种支架降解失重率和pH值,角接触测量仪检测2种支架亲水性。取3只出生2~5 d的白色新西兰乳兔,提取BMSCs,CCK-8法检测2种支架共培养细胞增殖活性,茜素红染色观察2种支架共培养细胞成骨分化情况。 结果 红外光谱检测证实成功制备出含有PLA、PTMC和β-Ca?(PO?)? 3种物质的复合支架。降解6~14周,与PLA/PTMC支架比较,PLA/PTMC-Ca?(PO?)? 支架在脂肪酶溶液和磷酸盐缓冲液(PBS)中的降解速率均明显升高(P<0.05或P<0.01);降解8~14周,与PLA/PTMC支架比较,PLA/PTMC-Ca?(PO?)? 支架在脂肪酶溶液中的pH值明显升高(P<0.01)。与PLA/PTMC支架比较,PLA/PTMC-Ca?(PO?)? 支架接触角明显减小(P<0.01)。细胞共培养第5和7天时,与PLA/PTMC支架比较,PLA/PTMC-Ca?(PO?)?支架共培养细胞增殖活性均明显升高(P<0.05或P<0.01)。共培养21 d后,2种支架与BMSCs重叠生长,局部均形成钙化结节,茜素红将其局部染成橘红色;与PLA/PTMC支架比较,PLA/PTMC-Ca?(PO?)? 支架共培养细胞矿化钙结节数量增多,密度增大,颜色加深。 结论 通过3D打印技术成功制备出含有PLA、PTMC和β-Ca?(PO?)? 的PLA/PTMC-Ca?(PO?)? 复合多孔支架,其降解性能良好,在生物相容性、亲水性和成骨诱导性等方面均表现出优势,是一种性能优良的骨缺损修复材料。
中图分类号:
- R331