吉林大学学报(医学版) ›› 2025, Vol. 51 ›› Issue (3): 576-589.doi: 10.13481/j.1671-587X.20250303
• 基础研究 • 上一篇
虫草素对RSL3诱导肝癌HepG2细胞铁死亡的增强作用及其机制
- 1.广西中医药大学研究生院,广西 南宁 530001
2.广西中医药大学附属瑞康医院肝内科,广西 南宁 530004
Improvement effect of cordycepin on ferroptosis in HepG2 cells induced by RSL3 and its mechanism
Han LIN1,Qiuyan YANG1,Jieyue ZHONG1,Bolun CHEN1,Wangxia TONG2( )
)
- 1.Graduate School,Guangxi University of Chinese Medicine,Guangxi 530001,China
2.Department of Medical Hepatology,Affiliated Ruikang Hospital,Guangxi University of Chinese Medicine,Nanning 530004,China
摘要:
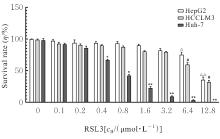
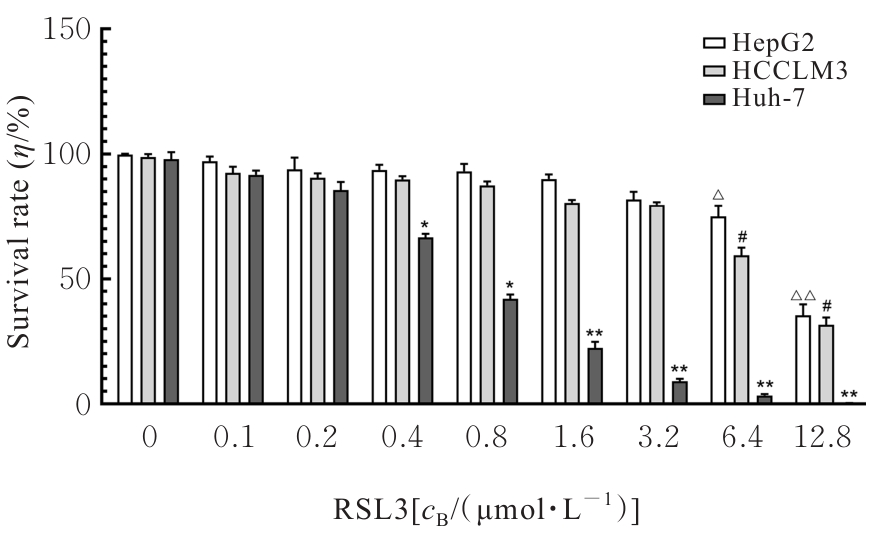
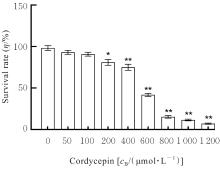
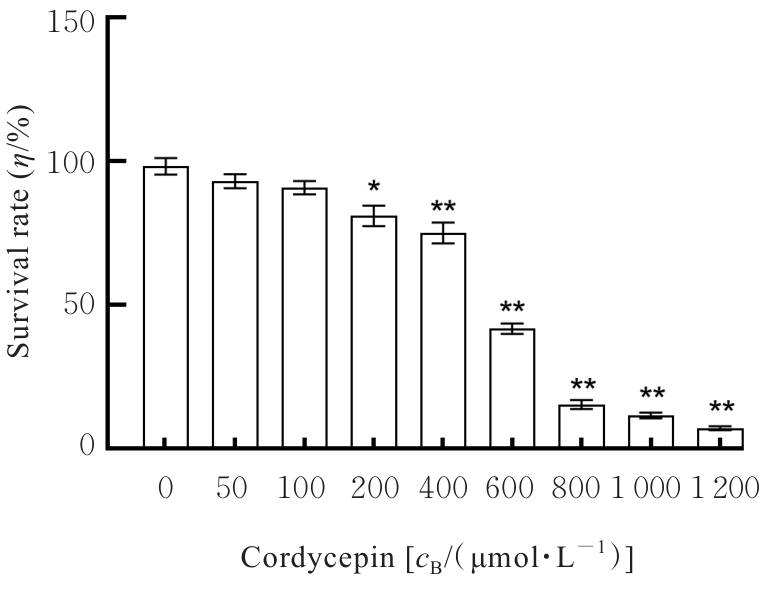
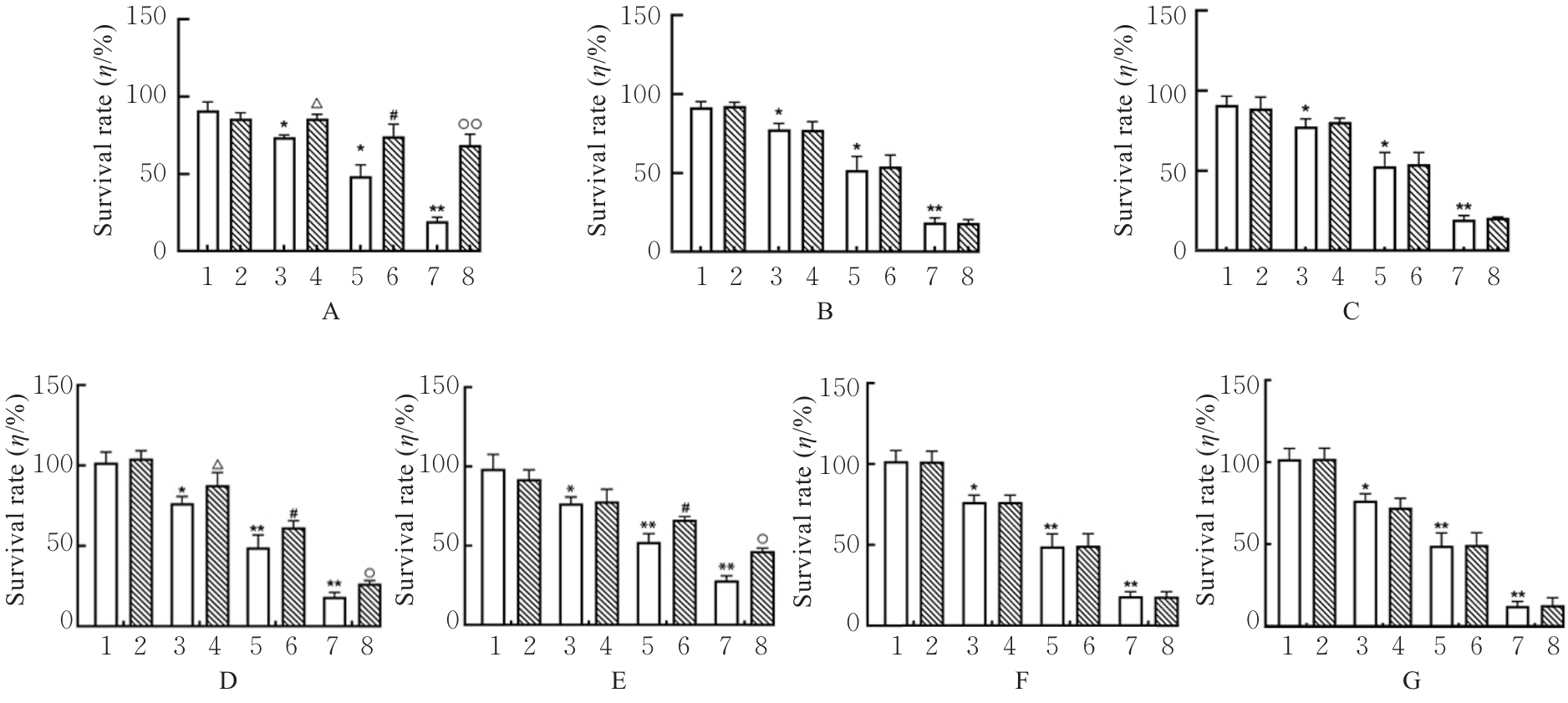
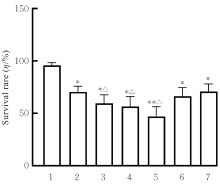
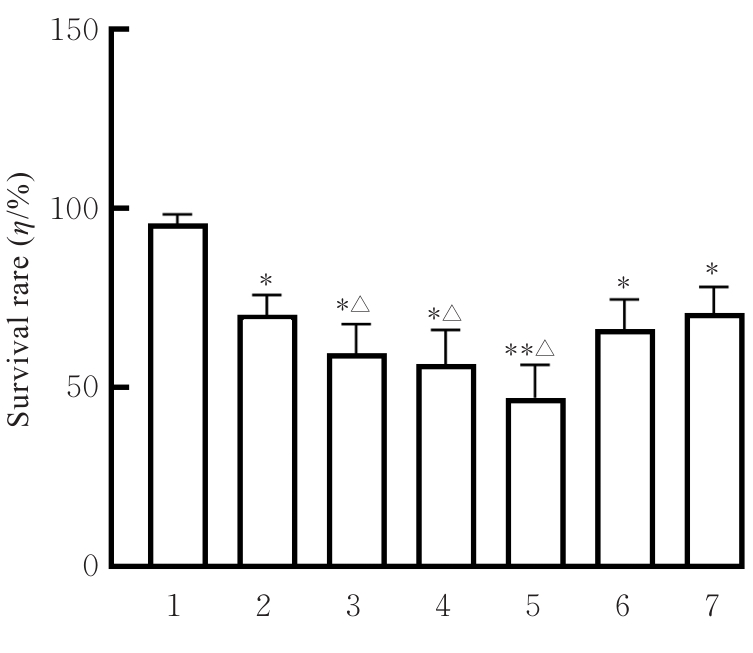
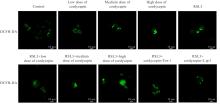
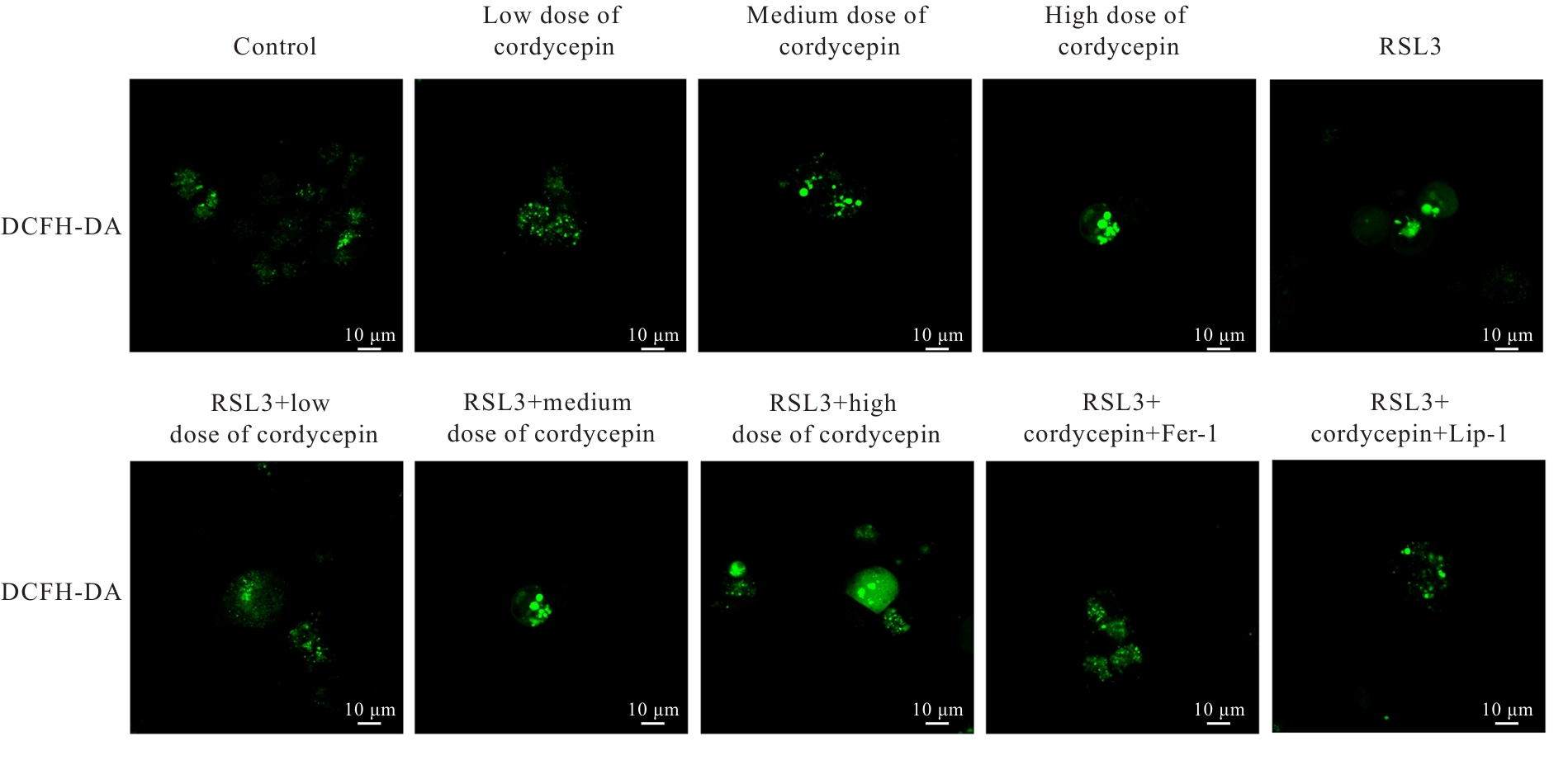
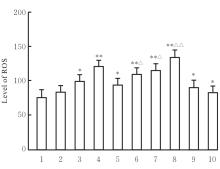
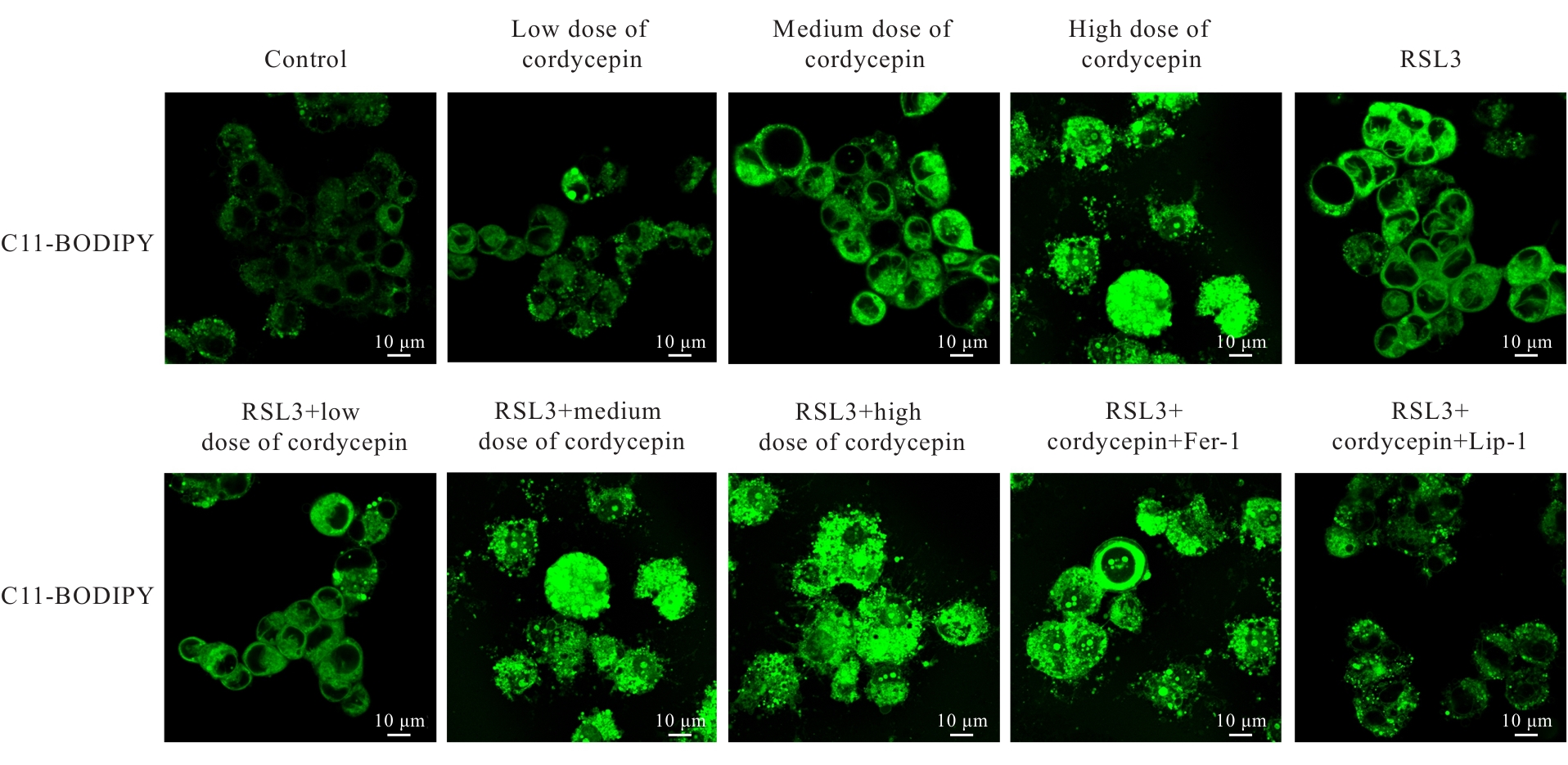
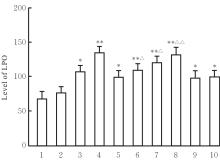
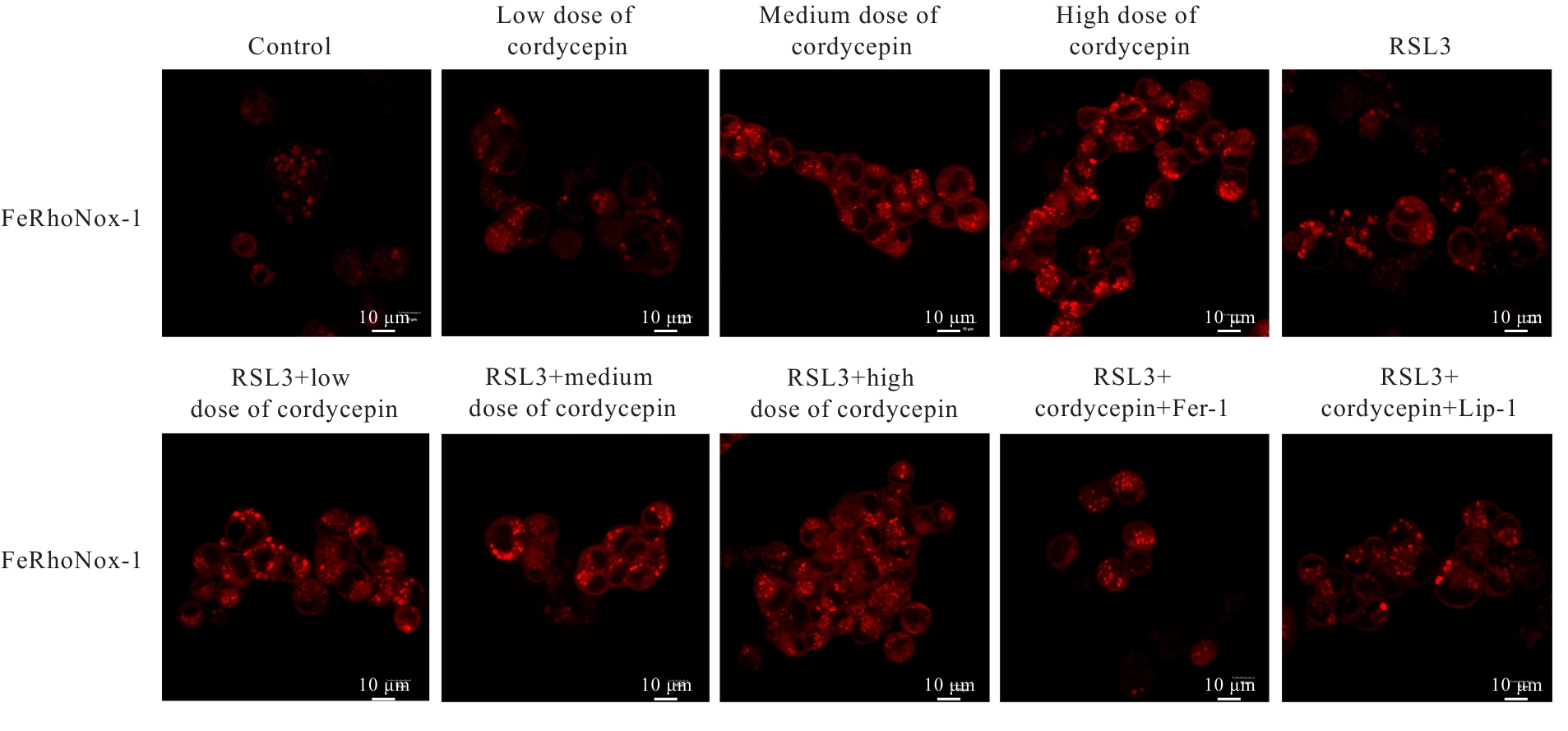
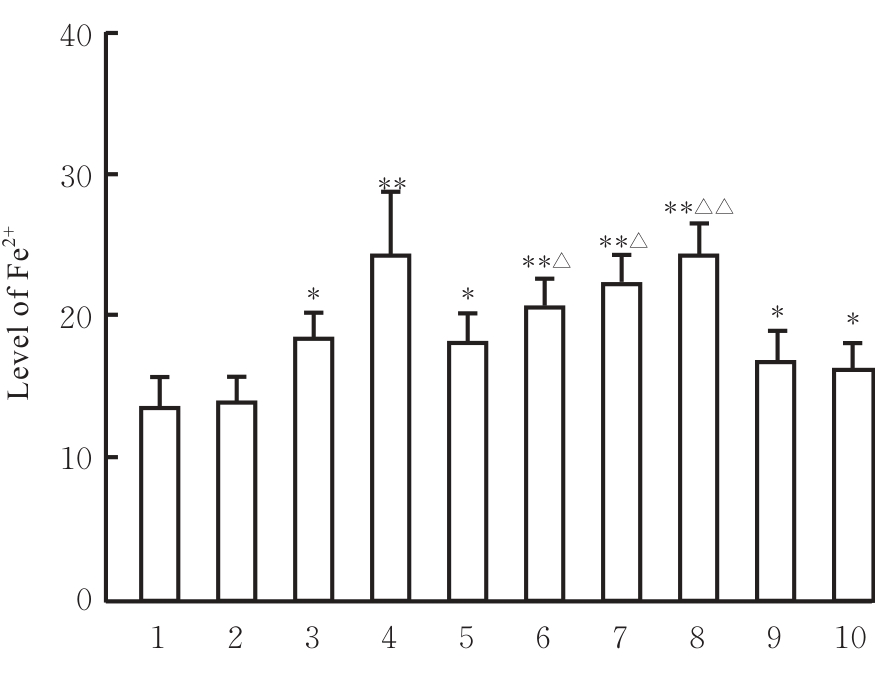
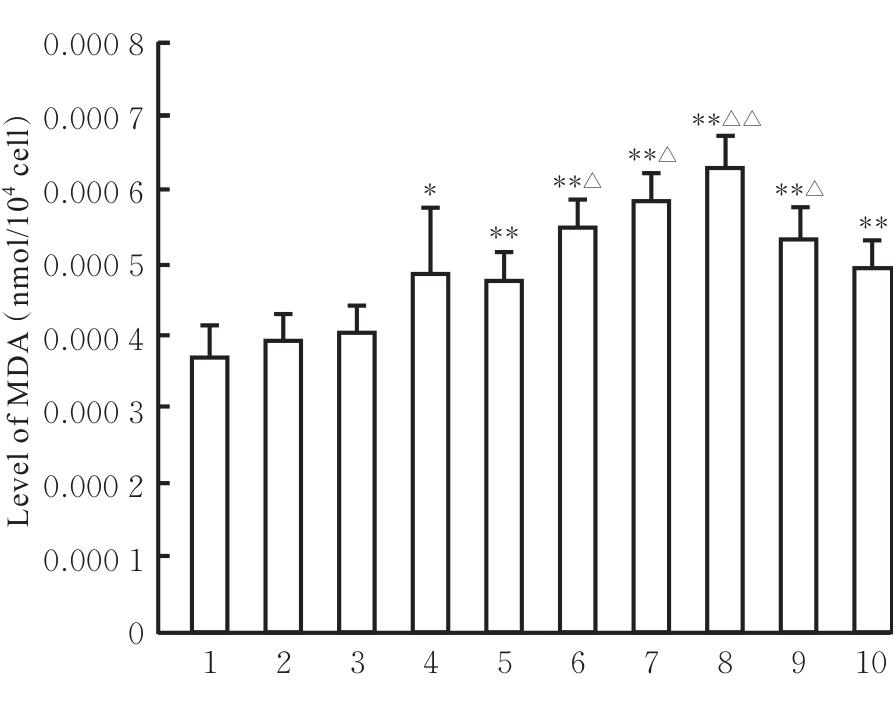
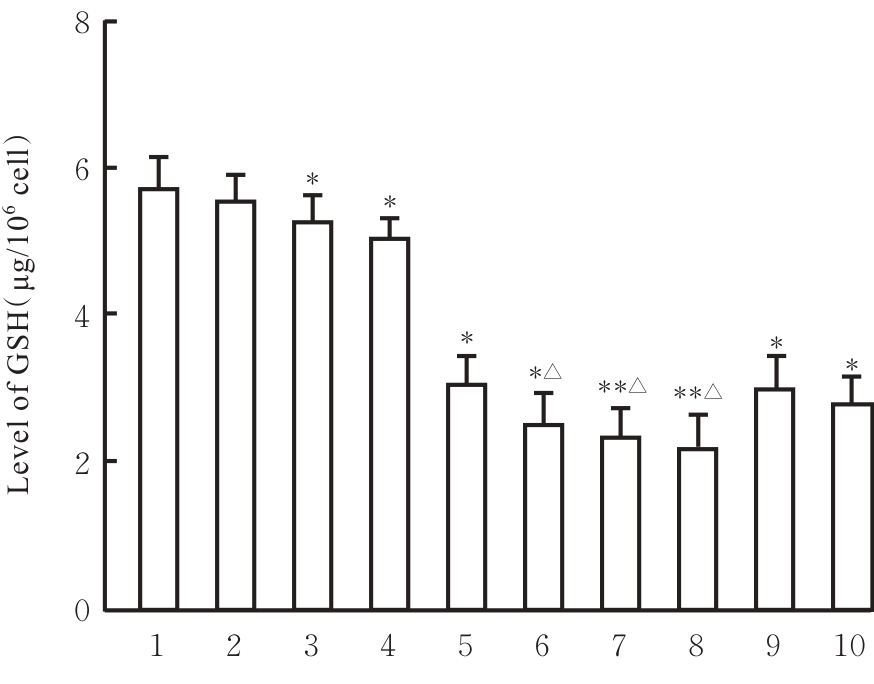
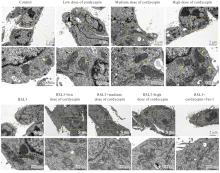
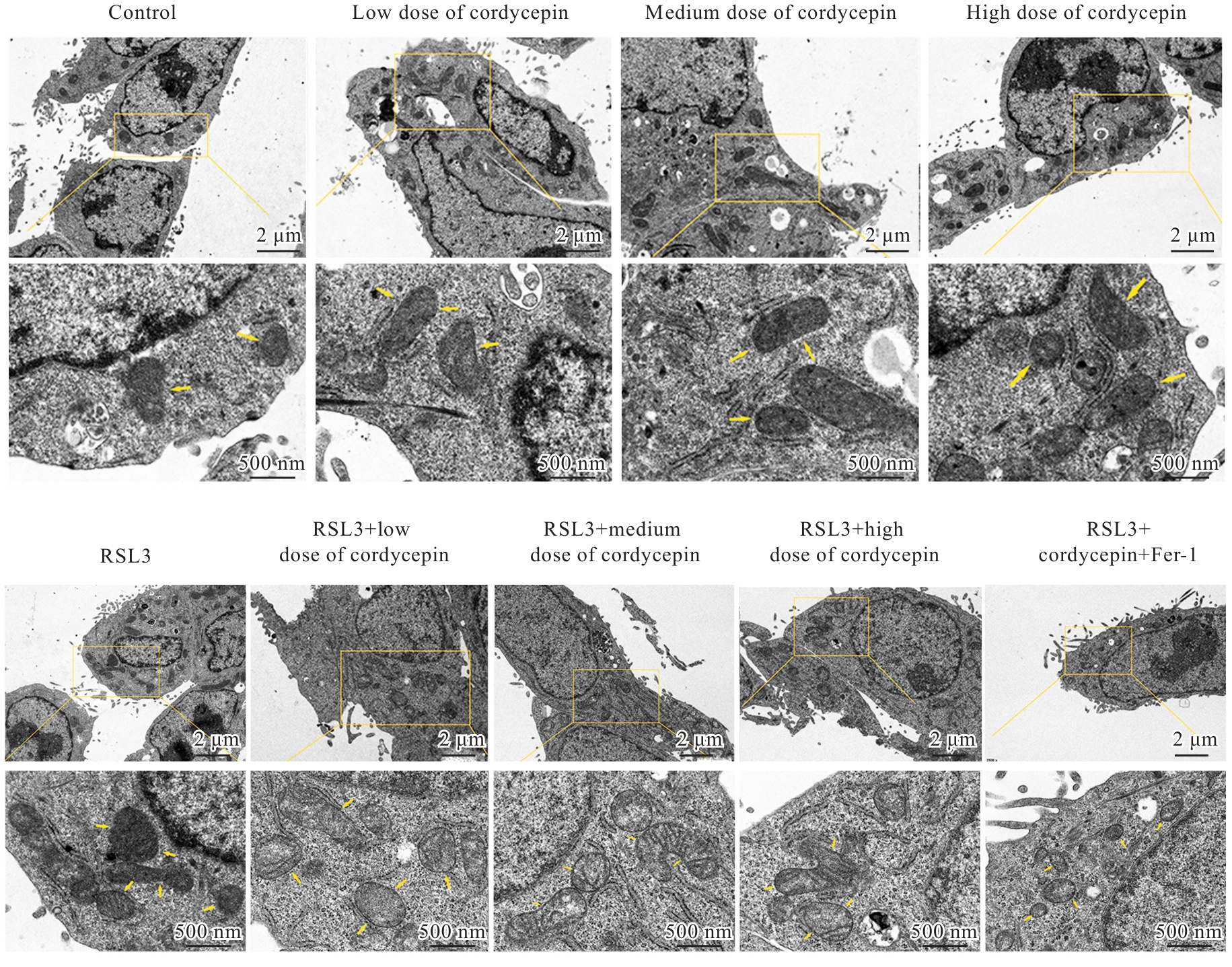
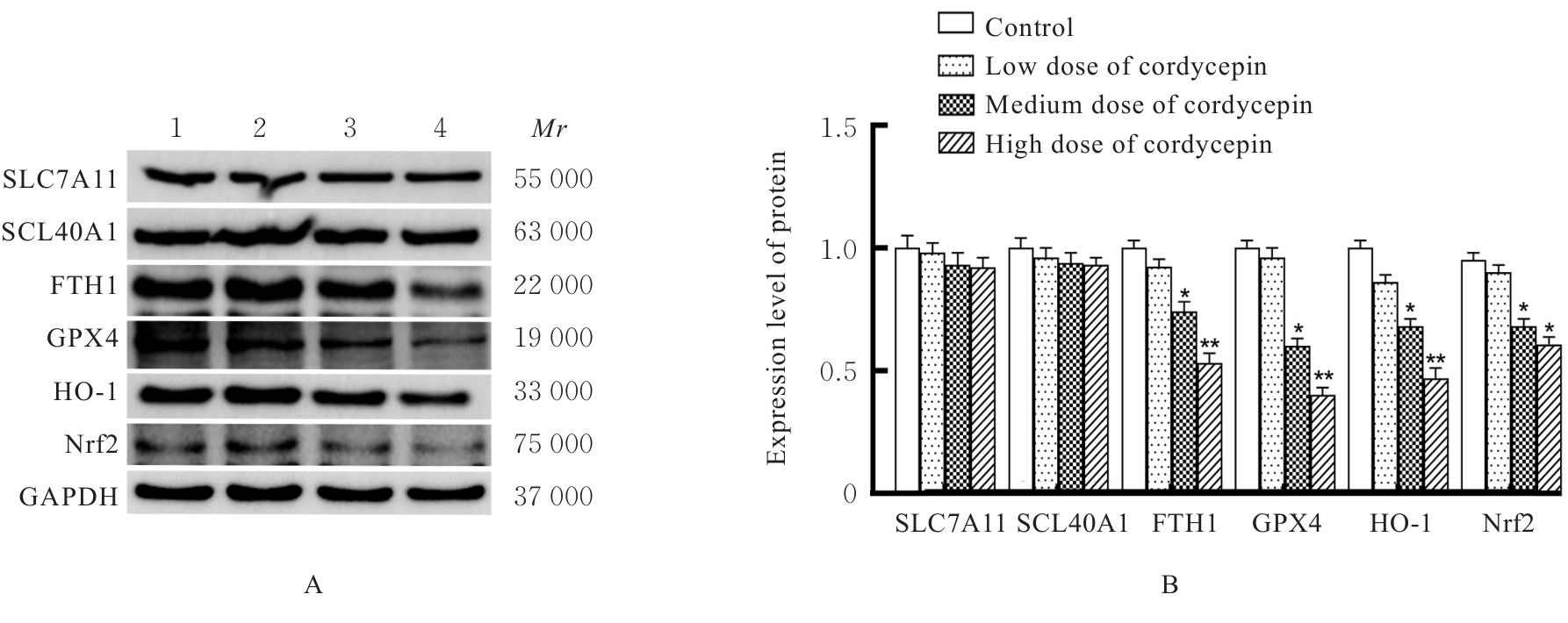
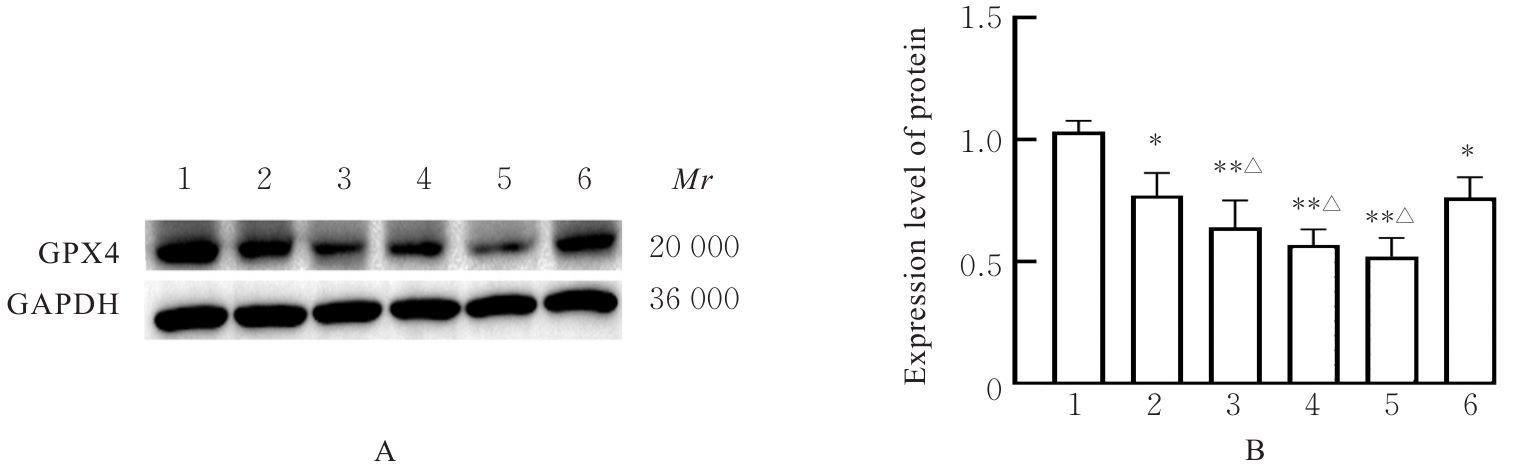
目的 探讨虫草素对铁死亡诱导剂RSL3诱导肝癌HepG2细胞铁死亡的增强作用,并阐明其潜在的作用机制。 方法 HepG2细胞分为对照组,RSL3组,低、中和高剂量虫草素组,RSL3组+低、中和高剂量虫草素组,RSL3+虫草素(中剂量)+铁死亡抑制剂Ferrostatin-1(Fer-1)组和RSL3+虫草素(中剂量)+铁死亡抑制剂Liproxstatin-1(Lip-1)组。采用0、1、5、10、15和20 μmol·L-1 RSL3分别干预HepG2、Huh-7及HCCLM3细胞24、48以及72 h,采用细胞计数试剂盒8(CCK-8)法检测各细胞活性,筛选RSL3最佳作用浓度和作用时间。分别采用0、50、100、200、400、600、800、1 000和1 200 μmol·L-1虫草素干预HepG2细胞24、48及72 h,采用CCK-8法检测细胞存活率,计算半数抑制浓度(IC50)值,筛选虫草素最佳作用浓度和作用时间。使用凋亡抑制剂Z-VAD-FMK、细胞自噬抑制剂Chloroquine(CQ)、细胞坏死性凋亡抑制剂Necrostatin-1(Nec-1)、Fer-1、Lip-1、Deferasirox和四甲基哌啶氧化物(TEMPO)分别干预HepG2细胞,计算HepG2细胞存活率。2',7'-二氯荧光素二乙酸酯(DCFH-DA)荧光探针法检测各组HepG2细胞中活性氧(ROS)水平,C11 BODIPY 581/591荧光探针法检测各组HepG2细胞中脂质过氧化(LPO)水平,FeRhoNox-1荧光探针法检测各组HepG2细胞中亚铁离子(Fe2+)水平,试剂盒检测HepG2细胞中谷胱甘肽(GSH)和丙二醛(MDA)水平,Western blotting法检测各组HepG2细胞中铁死亡相关蛋白、核转录因子E2相关因子2(Nrf2)和血红素氧合酶1(HO-1)蛋白表达水平,透射电镜观察各组HepG2细胞超微结构形态表现。 结果 CCK-8法,0.56 μmol·L-1 RSL3干预细胞时,3种细胞活性差异较明显。与0 μmol·L-1 RSL3组比较,6.4和12.8 μmol·L-1 RSL3组3种细胞存活率均明显降低(P<0.05)。HepG2细胞的IC50值最大,因此选择HepG2细胞进行后续实验。与0 μmol·L-1虫草素组比较,200、400、600、800、1 000和2 000 μmol·L-1虫草素组HepG2细胞存活率均明显降低(P<0.05或P<0.01)。选择0.5倍IC50值(267.9 μmol·L-1)、1倍IC50值(535.8 μmol·L-1)和1.5倍IC50值(803.7 μmol·L-1)分别作为低、中及高剂量虫草素组,干预时间为24 h。与对照组比较,低、中和高剂量虫草素组HepG2细胞存活率均明显降低(P<0.05或P<0.01)。与低、中和高剂量虫草素组比较,Z-VAD-FMK+低、中和高剂量虫草素组HepG2细胞存活率均明显升高(P<0.05或P<0.01),Fer-1+中和高剂量虫草素组及Lip-1+低、中和高剂量虫草素组HepG2细胞存活率均明显升高(P<0.05)。与对照组比较,RSL3组、RSL3+低、中和高剂量虫草素组、RSL3+虫草素+Fer-1组和RSL3+虫草素+Lip-1组HepG2细胞存活率均明显降低(P<0.05或P<0.01);与RSL3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞存活率均明显降低(P<0.05)。DCFH-DA荧光探针法,与对照组比较,中和高剂量虫草素组、RSL3组和RSL3+低、中和高剂量虫草素组细胞中ROS水平均明显升高(P<0.05或P<0.01);与RLS3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞中ROS水平均明显升高(P<0.05或P<0.01)。C11 BODIPY 581/591荧光探针法,与对照组比较,中和高剂量虫草素组、RSL3组和RSL3+低、中和高剂量虫草素组HepG2细胞中LPO水平均明显升高(P<0.05或P<0.01);与RSL3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞中LPO水平均明显升高(P<0.05或P<0.01)。FeRhoNox-1荧光探针法,与对照组比较,中和高剂量虫草素组、RSL3组和RSL3+低、中和高剂量虫草素组HepG2细胞中Fe2+水平均明显升高(P<0.05或P<0.01);与RSL3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞中Fe2+水平均明显升高(P<0.05或P<0.01)。与对照组比较,高剂量虫草素组、RSL3组和RSL3+低、中和高剂量虫草素组HepG2细胞中MAD水平均明显升高(P<0.05或P<0.01);与RSL3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞中MAD水平均明显升高(P<0.05或P<0.01)。与对照组比较,中和高剂量虫草素组、RSL3组和RSL3+低、中和高剂量虫草素组HepG2细胞中GSH水平均明显降低(P<0.05或P<0.01);与RSL3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞中GSH水平均明显降低(P<0.05)。与对照组比较,低和中剂量虫草素组HepG2细胞超微结构变化不明显,高剂量虫草素组部分细胞线粒体嵴减少,线粒体膜可见轻度肿胀和膜密度增高,线粒体膜中内膜结构轻度扭曲,RSL3组和RSL3+低、中及高剂量虫草素组均表现出铁死亡细胞超微结构变化;与RSL3组比较,RSL3+低、中和高剂量虫草素组表现为细胞线粒体膜破裂并伴有膜密度增高,线粒体膜中的内膜结构异常扭曲或扩张,线粒体嵴减少甚至消失。Western blotting法,与对照组比较,中和高剂量虫草素组HepG2细胞中FTH1和GPX4蛋白表达水平均明显降低(P<0.05或P<0.01),Nrf2和HO-1蛋白表达水平均明显降低(P<0.05或P<0.01),RSL3组和RSL3+虫草素+Fer-1组HepG2细胞中GPX4蛋白表达水平均明显降低(P<0.05或P<0.01);与RSL3组比较,RSL3+低、中和高剂量虫草素组HepG2细胞中GPX4蛋白表达水平均明显降低(P<0.05)。 结论 虫草素具有明显增强RSL3诱导肝癌HepG2细胞铁死亡的作用,并能够下调HepG2细胞中Nrf2和HO-1蛋白表达。
中图分类号:
- R735.7