吉林大学学报(医学版) ›› 2025, Vol. 51 ›› Issue (6): 1709-1716.doi: 10.13481/j.1671-587X.20250628
• 方法学 • 上一篇
SPHK1过表达慢病毒载体的构建和稳定转染SKOV3细胞系的建立
苏秋源1,赵玲1,谭佳佳1,莫世恩1,周海琴1,卢芳芳1,韦依1,周洋1,况燕1,2( )
)
- 1.广西医科大学第一附属医院妇科,广西 南宁 530021
2.广东省广州市第一人民医院妇科,广东 广州 510180
Construction of SPHK1 overexpression lentiviral vectors and establishment of stable transfected SKOV3 cell lines
Qiuyuan SU1,Ling ZHAO1,Jiajia TAN1,Shien MO1,Haiqin ZHOU1,Fangfang LU1,Yi WEI1,Yang ZHOU1,Yan KUANG1,2( )
)
- 1.Department of Gynecology,First Affiliated Hospital,Guangxi Medical University,Nanning 530021,China
2.Department of Gynecology,First People’s Hospital,Guangzhou City,Guangdong Provivce,Guangzhou 510180,China
摘要:
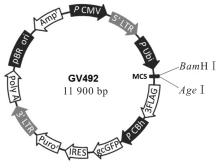
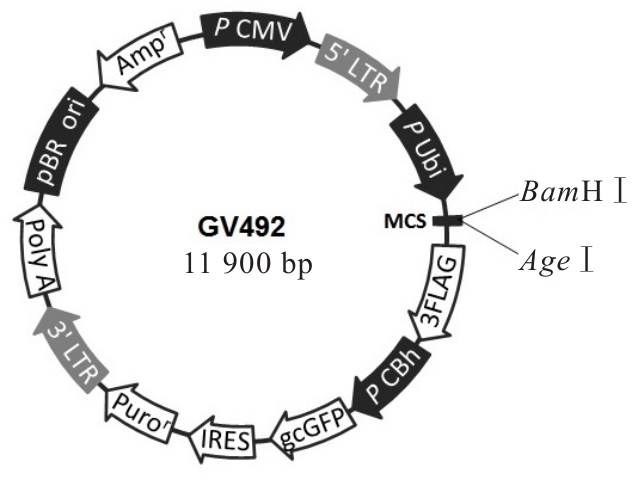
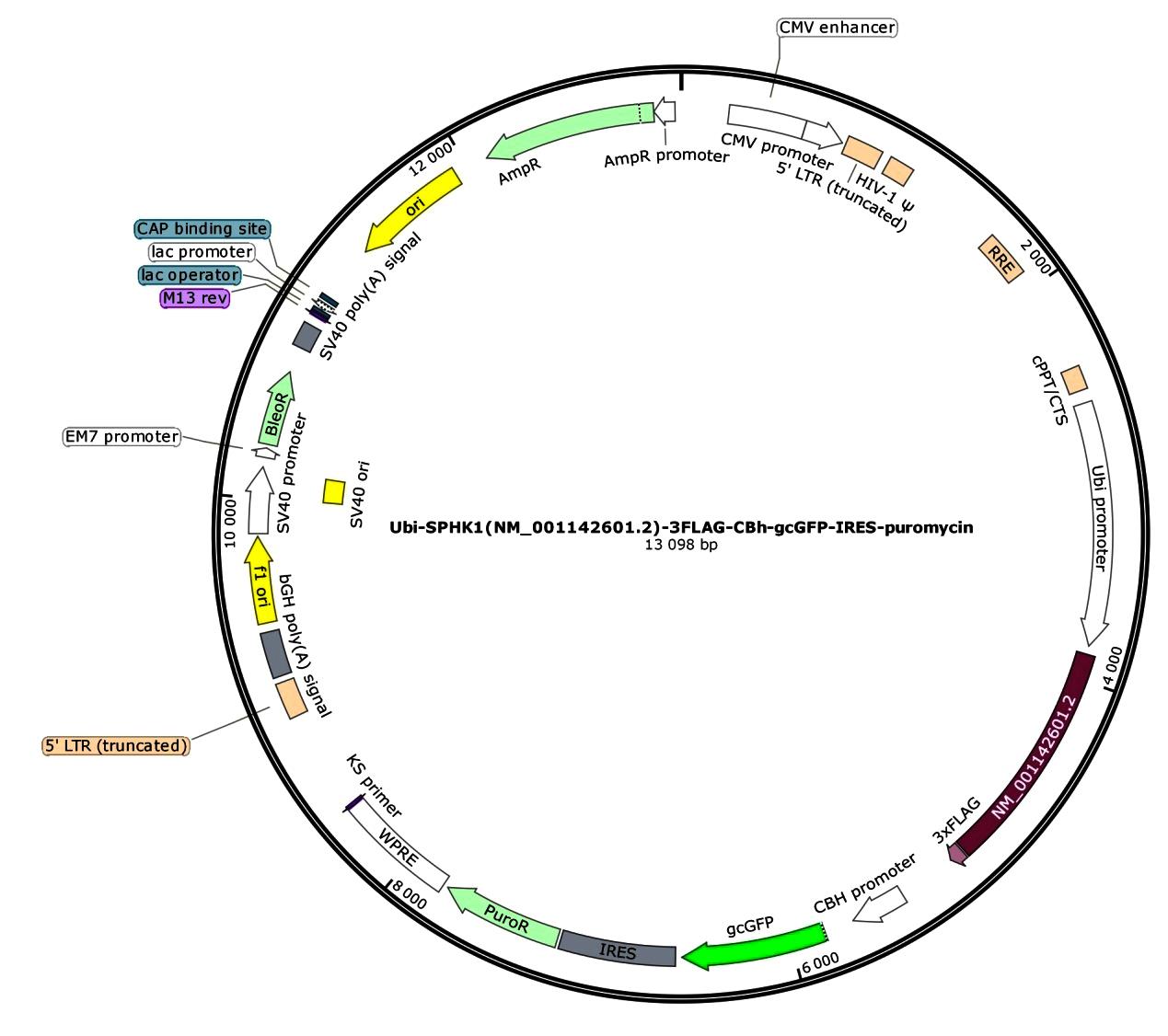
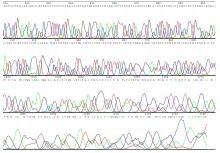
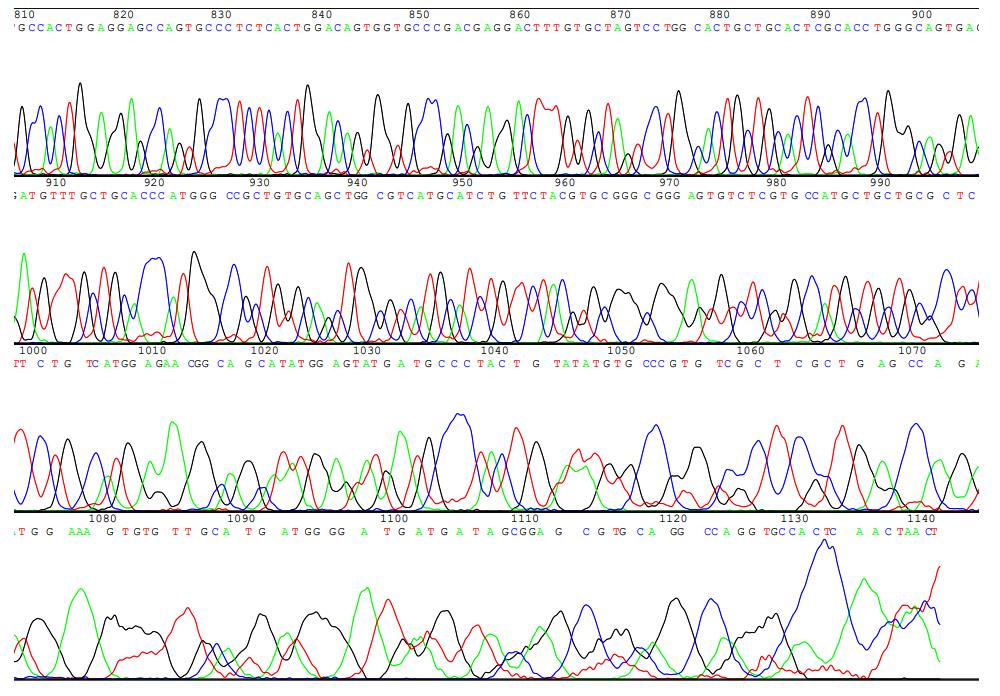
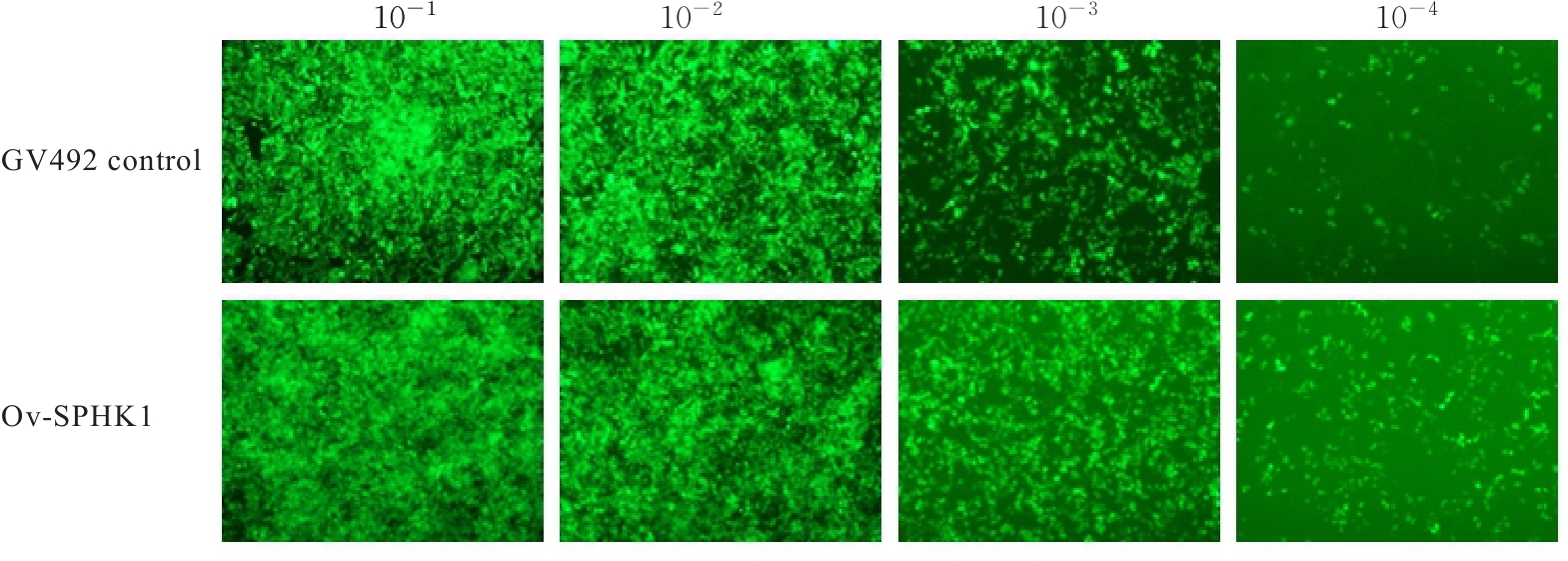
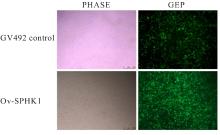
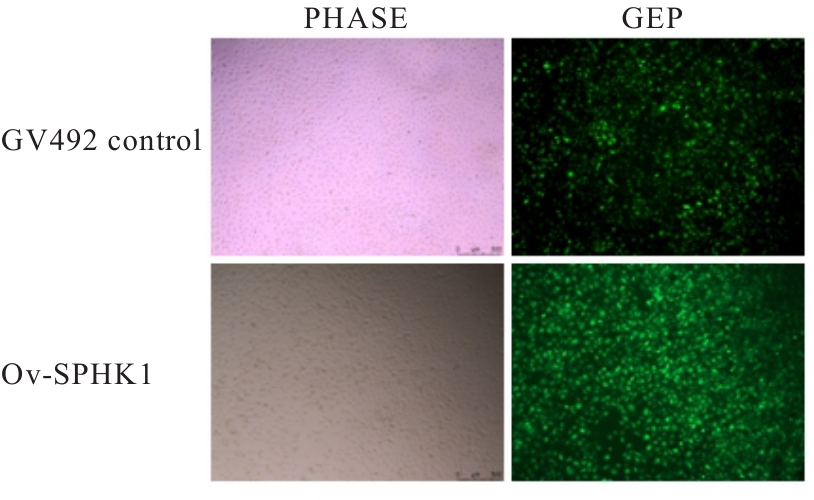
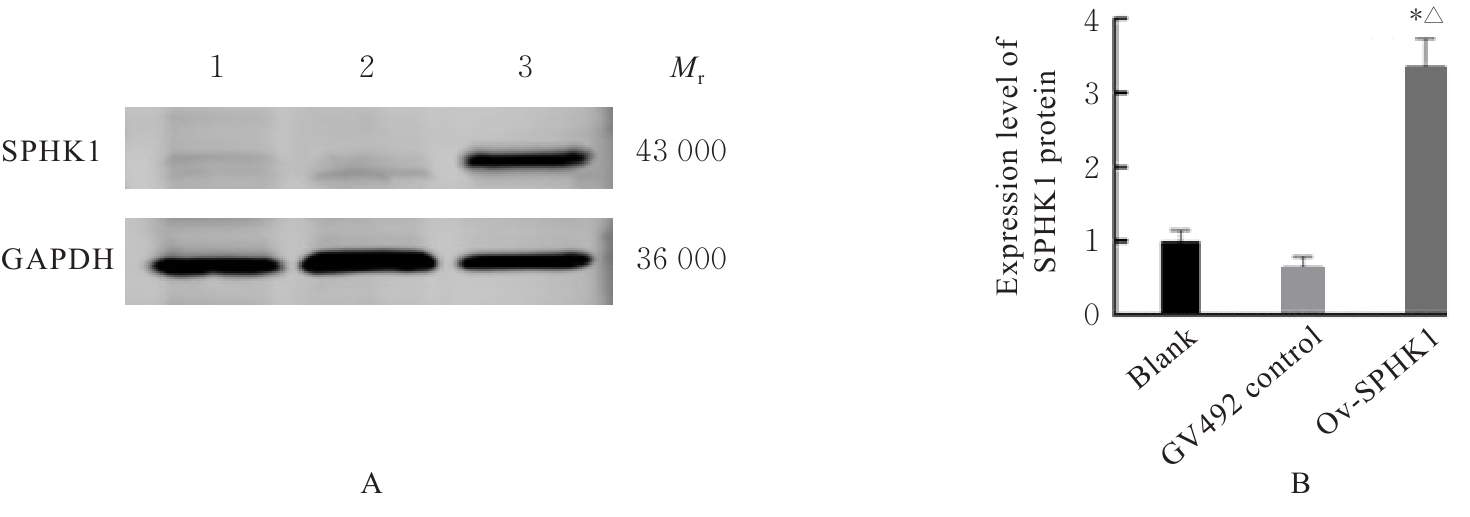
目的 构建鞘氨醇激酶1(SPHK1)过表达慢病毒载体,建立SKOV3慢病毒稳定转染细胞系。 方法 根据美国生物技术信息中心(NCBI)数据库提供的SPHK1的数据信息设计并合成引物,扩增目的基因,连接至经BamHⅠ和AgeⅠ限制性内切酶处理的GV492质粒,构建SPHK1过表达慢病毒载体,选取阳性克隆进行PCR和测序鉴定。将慢病毒质粒和慢病毒包装辅助质粒共同转染至HEK-293T细胞进行包装和滴度测定。根据测得的最佳感染复数(MOI)为10,将各组相应的慢病毒量转染至SKOV3细胞,分为空白组(不进行处理)、GV492对照组(GV492对照慢病毒感染SKOV3细胞)和GV492-SPHK1过表达组(GV492-SPHK1过表达慢病毒感染SKOV3细胞,ov-SPHK1组)。使用最佳浓度为2 mg·L-1的嘌呤霉素筛选稳定转染的SKOV3细胞系,48 h后换液,更换浓度为1 mg·L-1嘌呤霉素筛选14 d,荧光显微镜下观察细胞形态和荧光表达情况。采用实时荧光定量PCR(RT-qPCR)法检测各组SKOV3细胞中SPHK1 mRNA表达水平,Western blotting法检测各组SKOV3细胞中SPHK1蛋白表达水平。 结果 PCR测序,SPHK1过表达慢病毒载体基因序列与目标序列完全一致,成功构建SPHK1过表达慢病毒载体,GV492对照组和ov-SPHK1组慢病毒滴度分别为5×1011及8×1011 TU·L-1。GV492对照组和ov-SPHK1组SKOV3细胞状态良好,荧光表达强烈,提示成功构建SPHK1过表达的SKOV3稳定转染细胞系。RT-qPCR法检测,与空白组和GV492对照组比较,ov-SPHK1组SKOV3细胞中SPHK1 mRNA表达水平明显升高(P<0.01)。Western blotting法检测,与空白组和GV492对照组比较,ov-SPHK1组SKOV3细胞中SPHK1蛋白表达水平明显升高(P<0.01)。 结论 成功构建了SPHK1过表达慢病毒载体,并建立了稳定转染的SKOV3细胞。
中图分类号:
- R392