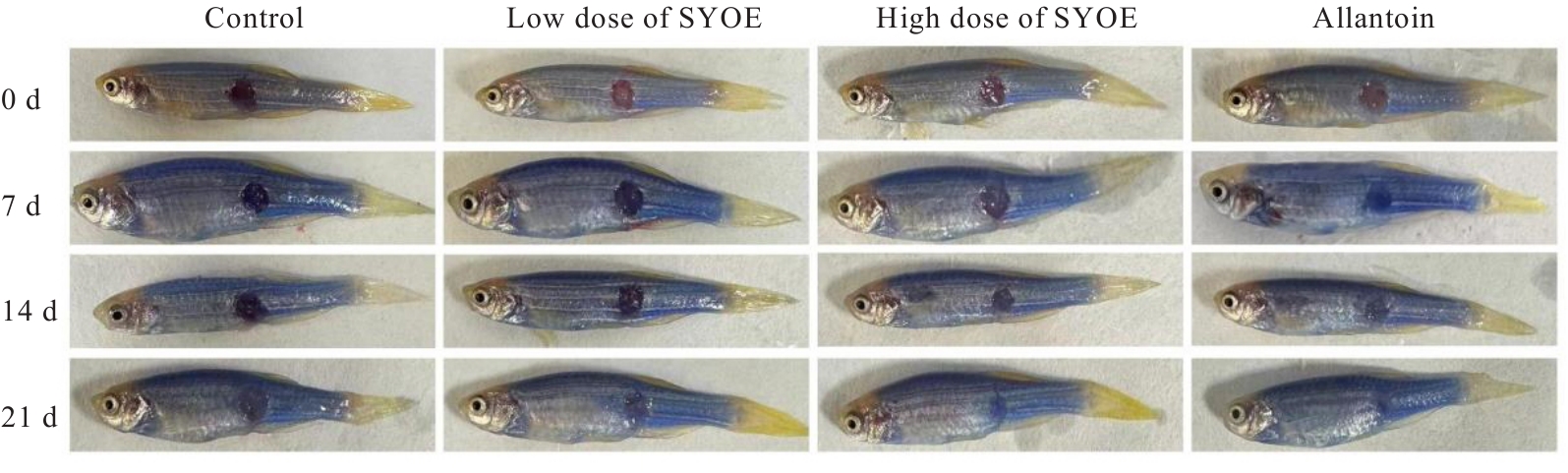
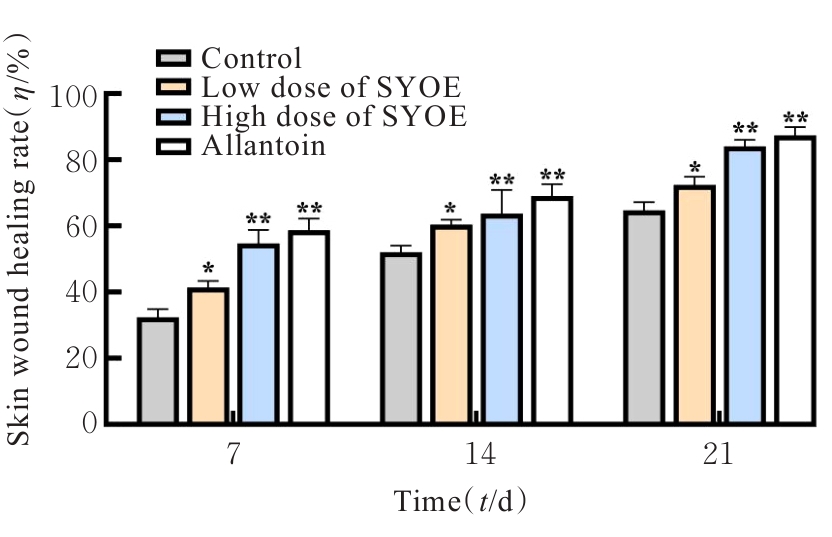
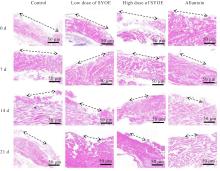
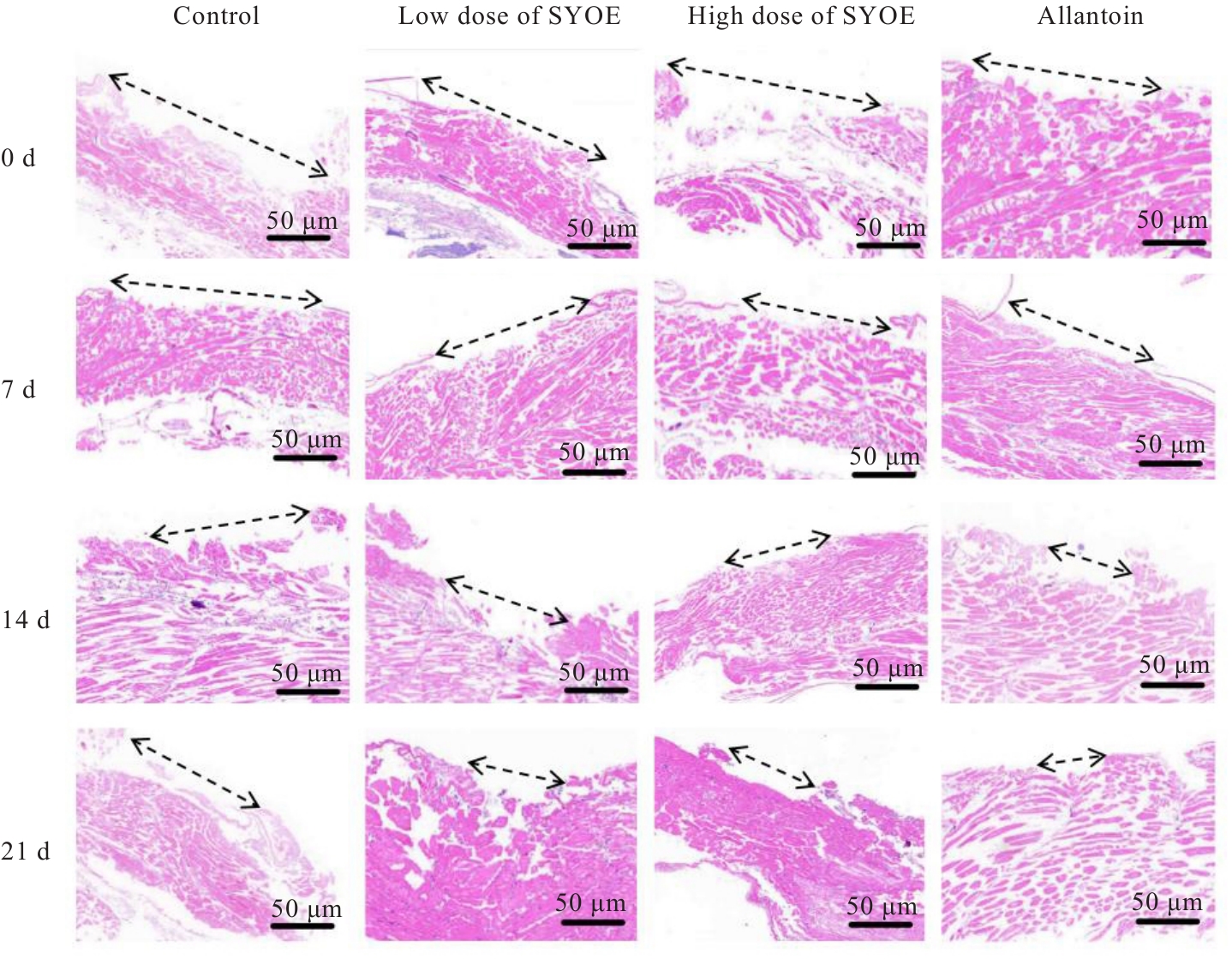
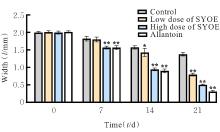
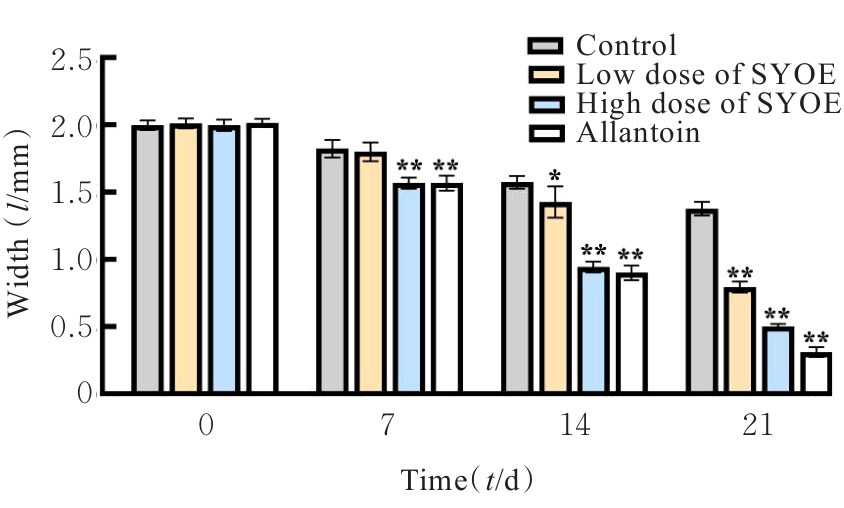
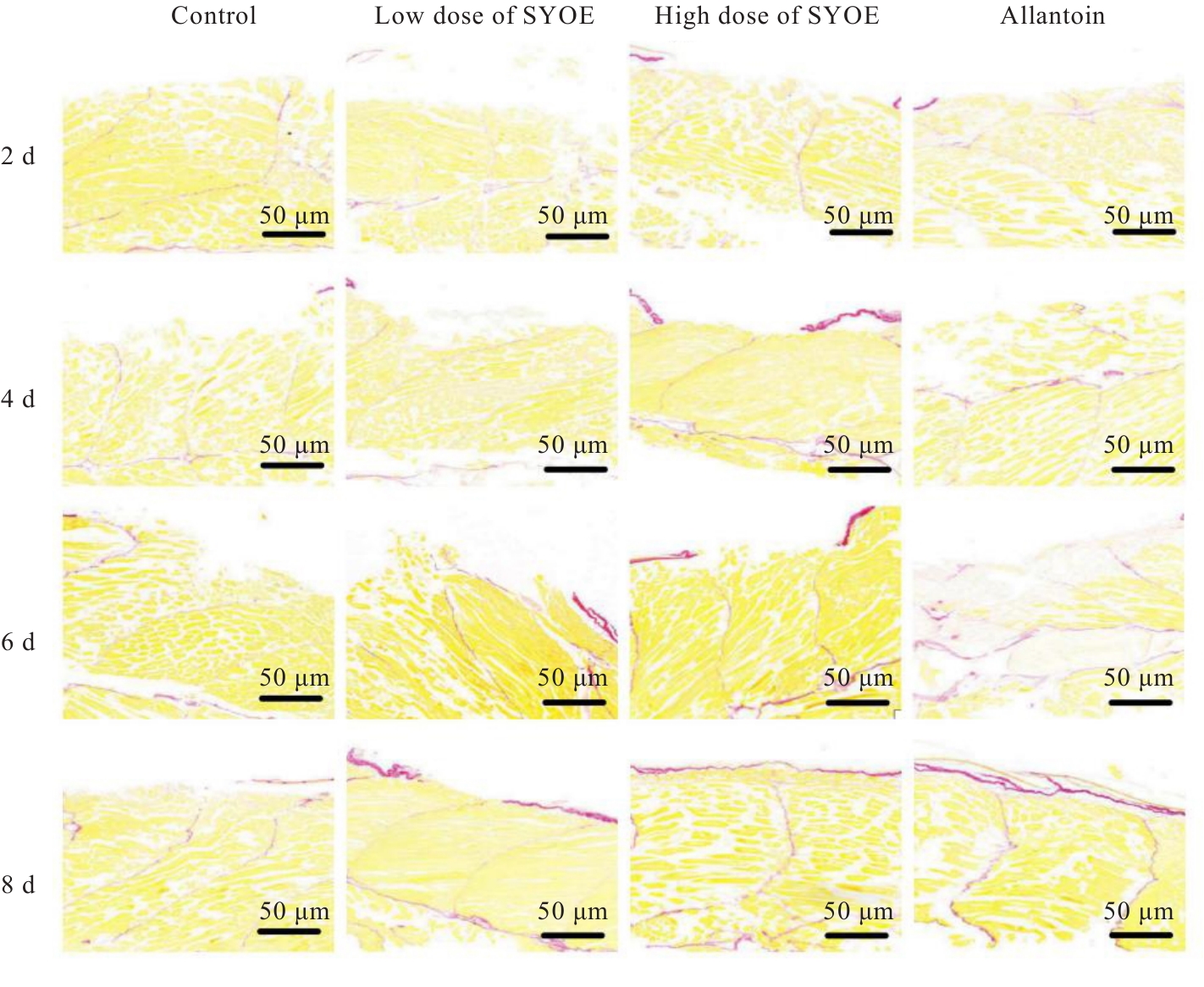
| [1] |
WU H F, LI Z T, TANG J Q, et al. The in vitro and in vivo anti-melanoma effects of hydroxyapatite nanoparticles: influences of material factors[J]. Int J Nanomedicine, 2019, 14: 1177-1191.
|
| [2] |
PERSINAL-MEDINA M, LLAMES S, CHACÓN M, et al. Polymerizable skin hydrogel for full thickness wound healing[J]. Int J Mol Sci, 2022, 23(9): 4837.
|
| [3] |
张 婧. 巨噬细胞在皮肤伤口愈合过程中的调控作用及其机制研究[D]. 西安: 西北大学, 2019.
|
| [4] |
YUAN M, LIU K, JIANG T, et al. GelMA/PEGDA microneedles patch loaded with HUVECs-derived exosomes and Tazarotene promote diabetic wound healing[J]. J Nanobiotechnology, 2022, 20(1): 147.
|
| [5] |
CAO C W, XIAO Z C, TONG H Q, et al. Oral intake of chicken bone collagen peptides anti-skin aging in mice by regulating collagen degradation and synthesis, inhibiting inflammation and activating lysosomes[J]. Nutrients, 2022, 14(8): 1622.
|
| [6] |
YU Q, SHEN Y X, XIAO F Q, et al. Yuhong ointment ameliorates inflammatory responses and wound healing in scalded mice[J]. J Ethnopharmacol, 2023, 306: 116118.
|
| [7] |
陈若岚, 薛慧琴, 丁 劲, 等. 艾灸联合生肌玉红膏对压疮大鼠皮肤损伤修复的作用机制[J]. 陕西中医, 2024, 45(8): 1042-1047.
|
| [8] |
YANG Y W, ZHOU Y W, GE M L. The effect of externally applied traditional Chinese medicine in diabetic foot: a systematic review and meta-analysis of 34 RCTs[J]. Foot, 2023, 56: 102045.
|
| [9] |
唐丽利, 冯文哲, 石 鹏, 等. 加味生肌玉红膏促进湿热证肛瘘术后创面愈合的实验研究[J]. 时珍国医国药, 2023, 34(8): 1798-1802.
|
| [10] |
刘 恒, 李爱茹, 高 岩, 等. 基于微环境生态观察生肌玉红膏对犬咬伤创面愈合的促进作用[J]. 现代中医临床, 2023, 30(6): 7-11.
|
| [11] |
GREENSPAN L J, AMEYAW K K, CASTRANOVA D, et al. Live imaging of cutaneous wound healing after rotary tool injury in zebrafish[J]. J Invest Dermatol, 2024, 144(4): 888-897.e6.
|
| [12] |
WILKINSON H N, IVESON S, CATHERALL P, et al. A novel silver bioactive glass elicits antimicrobial efficacy against Pseudomonas aeruginosa and Staphylococcus aureus in an ex vivo skin wound biofilm model[J]. Front Microbiol, 2018, 9: 1450.
|
| [13] |
EDIRISINGHE S L, RAJAPAKSHA D C, NIKAPITIYA C, et al. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish[J]. Fish Shellfish Immunol, 2020, 107: 414-425.
|
| [14] |
RICHARDSON R, SLANCHEV K, KRAUS C, et al. Adult zebrafish as a model system for cutaneous wound-healing research[J]. J Invest Dermatol, 2013, 133(6): 1655-1665.
|
| [15] |
XIE H J, BAI Q, KONG F K, et al. Allantoin-functionalized silk fibroin/sodium alginate transparent scaffold for cutaneous wound healing[J]. Int J Biol Macromol, 2022, 207: 859-872.
|
| [16] |
ZHU H C, AO H T, FU Y L, et al. Optimizing alginate dressings with allantoin and chemical modifiers to promote wound healing[J]. Int J Biol Macromol, 2024, 275(Pt 1): 133524.
|
| [17] |
CHEN Y X, ZHANG X R, LIU Z H, et al. Obstruction of the formation of granulation tissue leads to delayed wound healing after scald burn injury in mice[J]. Burns Trauma, 2021, 9: tkab004.
|
| [18] |
HE S Q, WALIMBE T, CHEN H Y, et al. Bioactive extracellular matrix scaffolds engineered with proangiogenic proteoglycan mimetics and loaded with endothelial progenitor cells promote neovascularization and diabetic wound healing[J]. Bioact Mater, 2021, 10: 460-473.
|
| [19] |
ZHOU W C, ZI L, CEN Y, et al. Copper sulfide nanoparticles-incorporated hyaluronic acid injectable hydrogel with enhanced angiogenesis to promote wound healing[J]. Front Bioeng Biotechnol, 2020, 8: 417.
|
| [20] |
ZHAO R L, JIN X Y, LI A, et al. Precise diabetic wound therapy: PLS nanospheres eliminate senescent cells via DPP4 targeting and PARP1 activation[J]. Adv Sci, 2022, 9(1): 2104128.
|
| [21] |
YANG X Y, ZHI X Y, SONG Z L, et al. Flesh quality of hybrid grouper (Epinephelus fuscoguttatus ♀× Epinephelus lanceolatus ♂) fed with hydrolyzed porcine mucosa-supplemented low fishmeal diet[J]. Anim Nutr, 2022, 8(1): 114-124.
|
| [22] |
XIE Y J, QIAO K, YUE L N, et al. A self-crosslinking, double-functional group modified bacterial cellulose gel used for antibacterial and healing of infected wound[J]. Bioact Mater, 2022, 17: 248-260.
|
| [23] |
CUI H S, JOO S Y, CHO Y S, et al. Effect of combining low temperature plasma, negative pressure wound therapy, and bone marrow mesenchymal stem cells on an acute skin wound healing mouse model[J]. Int J Mol Sci, 2020, 21(10): 3675.
|
| [24] |
NAGASAKI K, NAKASHIMA A, TAMURA R, et al. Mesenchymal stem cells cultured in serum-free medium ameliorate experimental peritoneal fibrosis[J]. Stem Cell Res Ther, 2021, 12(1): 203.
|
| [25] |
WU S Y, CHEN Y T, TSAI G Y, et al. Protective effect of low-molecular-weight fucoidan on radiation-induced fibrosis through TGF-β1/Smad pathway-mediated inhibition of collagen I accumulation[J]. Mar Drugs, 2020, 18(3): 136.
|
| [26] |
邵世清, 曲长萍, 李好山, 等. 脂联素干预对宫腔粘连大鼠子宫内膜组织中NLRP3、TGF-β1和Smad2表达的影响[J]. 郑州大学学报(医学版), 2024, 59(6): 777-782.
|
| [27] |
KIM H G, LIM Y S, HWANG S, et al. Di-(2-ethylhexyl) phthalate triggers proliferation, migration, stemness, and epithelial-mesenchymal transition in human endometrial and endometriotic epithelial cells via the transforming growth factor-β/Smad signaling pathway[J]. Int J Mol Sci, 2022, 23(7): 3938.
|
| [28] |
PENG Y, WU S, TANG Q Y, et al. KGF-1 accelerates wound contraction through the TGF-β1/Smad signaling pathway in a double-paracrine manner[J]. J Biol Chem, 2019, 294(21): 8361-8370.
|
| [29] |
HAU H T A, OGUNDELE O, HIBBERT A H, et al. Maternal Larp6 controls oocyte development, chorion formation and elevation[J]. Development, 2020, 147(4): dev187385.
|
| [30] |
PALUNGWACHIRA P, TANCHAROEN S, PHRUKSANIYOM C, et al. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts[J]. Oxid Med Cell Longev, 2019, 2019: 2089817.
|
| [31] |
LEE J J, NG S C, NI Y T, et al. Protective effects of galangin against H2O2/UVB-induced dermal fibroblast collagen degradation via hsa-microRNA-4535-mediated TGFβ/Smad signaling[J]. Aging, 2021, 13(23): 25342-25364.
|
| [32] |
CHENG Y T, LI Y F, HUANG S Y, et al. Hybrid freeze-dried dressings composed of epidermal growth factor and recombinant human-like collagen enhance cutaneous wound healing in rats[J]. Front Bioeng Biotechnol, 2020, 8: 742.
|
 ),Jianzeng LIU3(
),Jianzeng LIU3( )
)