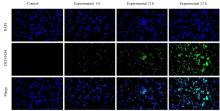
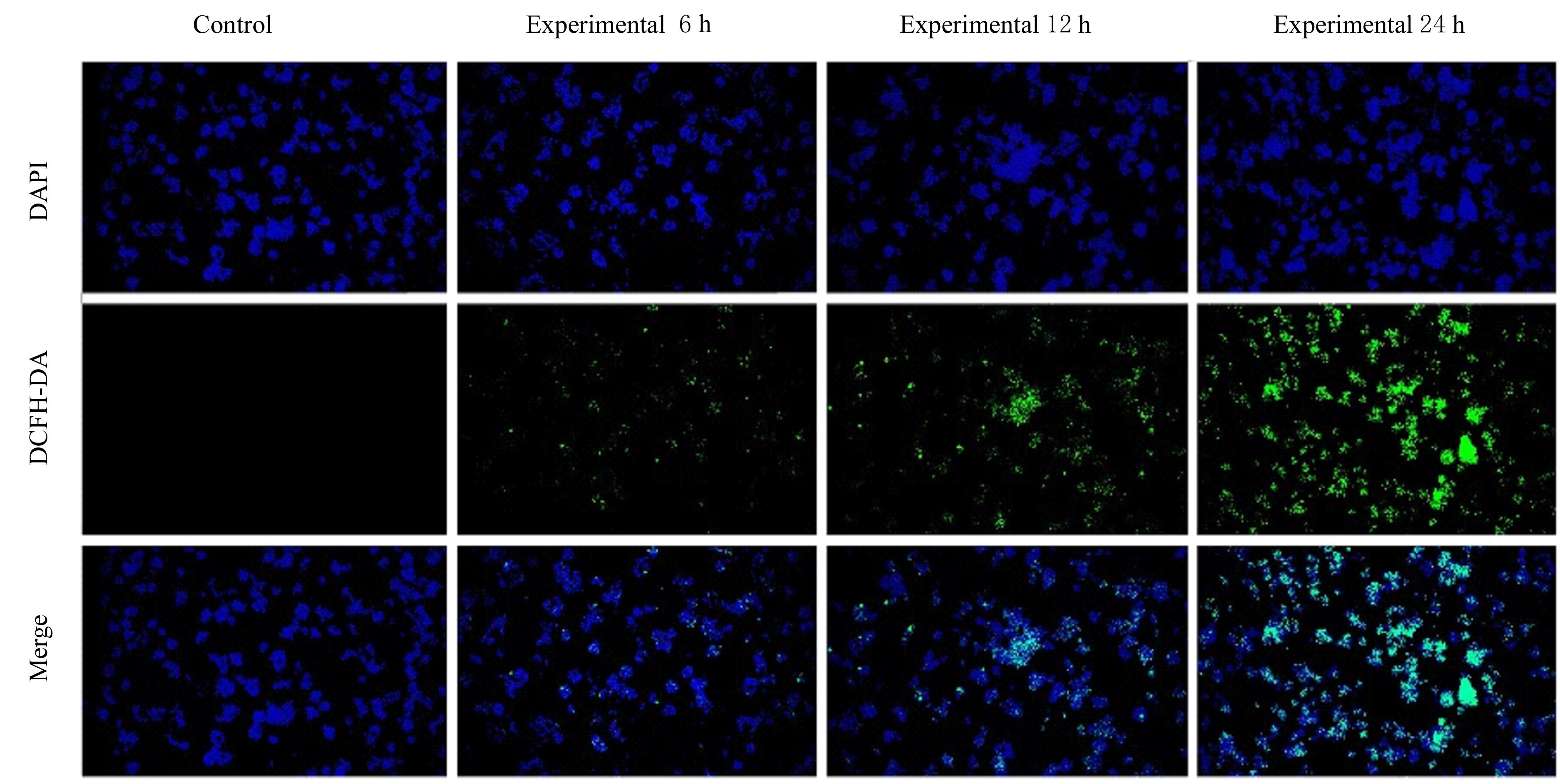
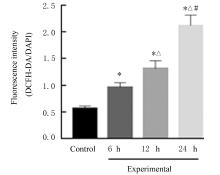
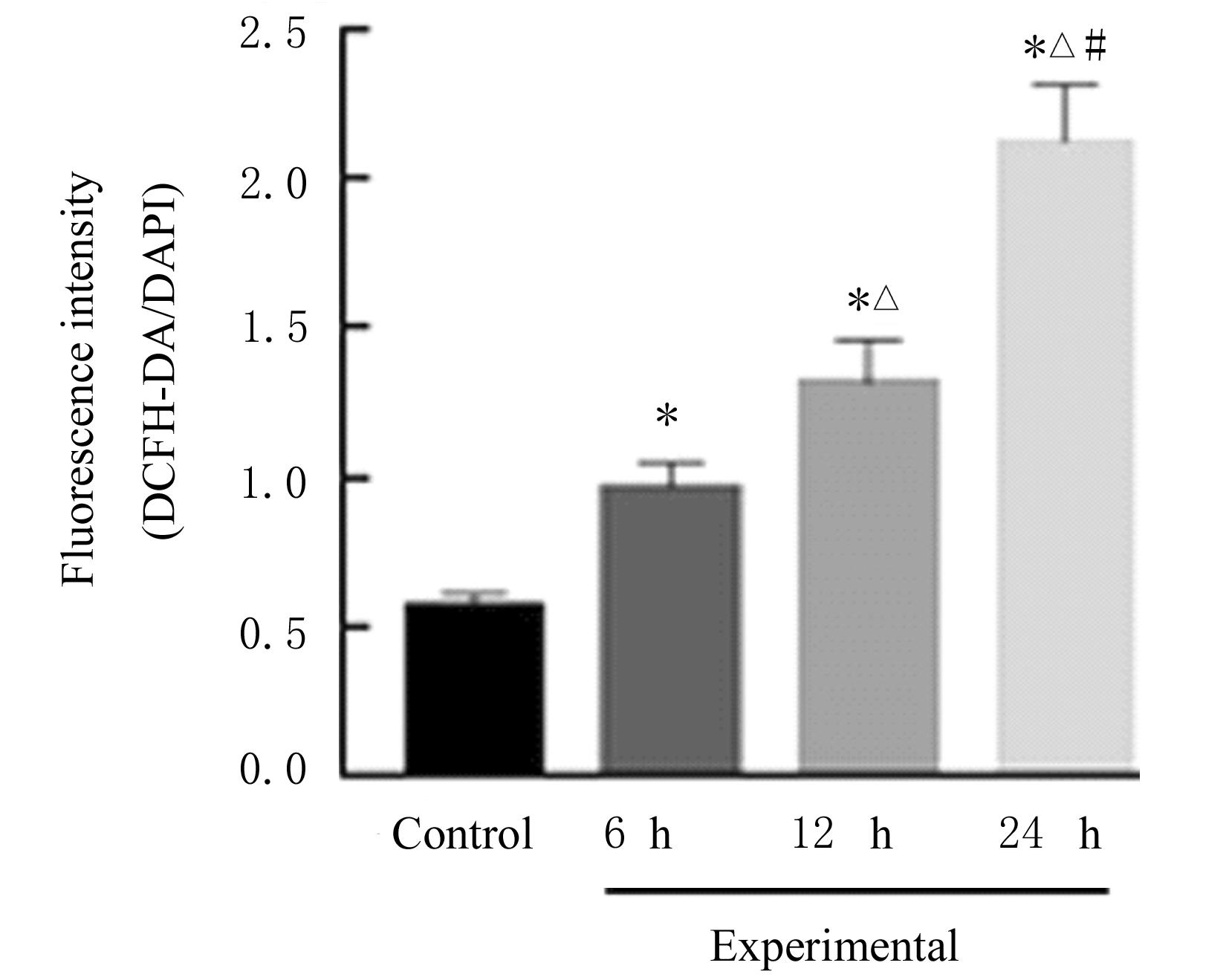
| 1 |
JIAO J, JING W D, SI Y, et al. The prevalence and severity of periodontal disease in Mainland China: data from the Fourth National Oral Health Survey (2015-2016)[J]. J Clin Periodontol, 2021, 48(2): 168-179.
|
| 2 |
SLOTS J. Periodontitis: facts, fallacies and the future[J]. Periodontol 2000, 2017, 75(1): 7-23.
|
| 3 |
XU W Z, ZHOU W, WANG H Z, et al. Roles of porphyromonas gingivalis and its virulence factors in periodontitis[J]. Adv Protein Chem Struct Biol, 2020, 120: 45-84.
|
| 4 |
ORECCHIONI M, GHOSHEH Y, PRAMOD A B,et al.Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages[J]. Front Immunol, 2019, 10: 1084.
|
| 5 |
HIRSCHHORN T, STOCKWELL B R. The development of the concept of ferroptosis[J]. Free Radic Biol Med, 2019, 133: 130-143.
|
| 6 |
XU T, DING W, JI X Y, et al. Molecular mechanisms of ferroptosis and its role in cancer therapy[J]. J Cell Mol Med, 2019, 23(8): 4900-4912.
|
| 7 |
LI J C, LU K M, SUN F L, et al. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway[J]. J Transl Med, 2021, 19: 96.
|
| 8 |
WEI S S, BI J B, YANG L F, et al. Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice[J]. Clin Transl Med, 2020, 10(5): e173.
|
| 9 |
ZHAO Y H, LI J, GUO W, et al. Periodontitis-level butyrate-induced ferroptosis in periodontal ligament fibroblasts by activation of ferritinophagy[J]. Cell Death Discov, 2020, 6(1): 119.
|
| 10 |
GUO W, ZHAO Y H, LI H X, et al. NCOA4-mediated ferritinophagy promoted inflammatory responses in periodontitis[J]. J Periodontal Res, 2021, 56(3): 523-534.
|
| 11 |
LI N, WANG W, ZHOU H, et al. Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury[J]. Free Radic Biol Med, 2020, 160: 303-318.
|
| 12 |
LIU P F, FENG Y T, LI H W, et al. Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis[J]. Cell Mol Biol Lett, 2020, 25: 10.
|
| 13 |
PRESHAW P M, SEYMOUR R A, HEASMAN P A. Current concepts in periodontal pathogenesis[J]. Dent Update, 2004, 31(10): 570-572, 574-578.
|
| 14 |
ALMUBARAK A, TANAGALA K K K, PAPAPANOU P N, et al. Disruption of monocyte and macrophage homeostasis in periodontitis[J]. Front Immunol, 2020, 11: 330.
|
| 15 |
MAO H M, ZHAO Y H, LI H X, et al. Ferroptosis as an emerging target in inflammatory diseases[J]. Prog Biophys Mol Biol, 2020, 155: 20-28.
|
| 16 |
MARTINET W, COORNAERT I, PUYLAERT P, et al. Macrophage death as a pharmacological target in atherosclerosis[J]. Front Pharmacol, 2019, 10: 306.
|
| 17 |
WANG Y, QUAN F, CAO Q H, et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis[J]. J Adv Res, 2020, 28: 231-243.
|
| 18 |
LI J, SHI J H, PAN Y, et al. Transcription modulation by CDK9 regulates inflammatory genes and RIPK3-MLKL-mediated necroptosis in periodontitis progression[J]. Sci Rep, 2019, 9(1): 17369.
|
| 19 |
LUCAS H, BARTOLD P M, DHARMAPATNI A A S S K, et al. Inhibition of apoptosis in periodontitis[J]. J Dent Res, 2010, 89(1): 29-33.
|
| 20 |
DIXON S J, LEMBERG K M, LAMPRECHT M R, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death[J]. Cell, 2012, 149(5): 1060-1072.
|
| 21 |
TANG D L, KROEMER G. Ferroptosis[J]. Curr Biol, 2020, 30(21): R1292-R1297.
|
| 22 |
ČEPELAK I, DODIG S, DODIG D Č. Ferroptosis: regulated cell death[J]. Arh Hig Rada Toksikol, 2020, 71(2): 99-109.
|
| 23 |
YUAN H, LI X M, ZHANG X Y, et al. Identification of ACSL4 as a biomarker and contributor of ferroptosis[J]. Biochem Biophys Res Commun, 2016, 478(3): 1338-1343.
|
| 24 |
DOLL S, PRONETH B, TYURINA Y Y, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition[J]. Nat Chem Biol, 2017, 13(1): 91-98.
|
| 25 |
SEIBT T M, PRONETH B, CONRAD M. Role of GPX4 in ferroptosis and its pharmacological implication[J].Free Radic Biol Med,2019,133:144-152.
|
| 26 |
FENG H Z, SCHORPP K, JIN J, et al. Transferrin receptor is a specific ferroptosis marker[J]. Cell Rep, 2020, 30(10): 3411-3423.e7.
|
| 27 |
PUDLA M, SRISATJALUK R, UTAISINCHAROEN P. Induction of inducible nitric oxide synthase (iNOS) in Porphyromonas gingivalis LPS-treated mouse macrophage cell line (RAW264.7) requires Toll-like receptor 9[J]. Inflamm Res, 2018, 67(9): 723-726.
|
| 28 |
XIE Y, HOU W, SONG X, et al. Ferroptosis: process and function[J].Cell Death Differ,2016,23(3):369-379.
|
| 29 |
BAŇASOVÁ L, KAMODYOVÁ N, JANŠÁKOVÁ K, et al. Salivary DNA and markers of oxidative stress in patients with chronic periodontitis[J]. Clin Oral Investig, 2015, 19(2): 201-207.
|
| 30 |
LI W Y, LI W, LENG Y, et al. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress[J]. DNA Cell Biol, 2020, 39(2): 210-225.
|
| 31 |
STAMENKOVIC A, PIERCE G N, RAVANDI A. Phospholipid oxidation products in ferroptotic myocardial cell death[J]. Am J Physiol Heart Circ Physiol, 2019, 317(1): H156-H163.
|
| 32 |
GENG N, SHI B J, LI S L, et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells[J]. Eur Rev Med Pharmacol Sci, 2018, 22(12): 3826-3836.
|
| 33 |
VALKO M, LEIBFRITZ D, MONCOL J, et al. Free radicals and antioxidants in normal physiological functions and human disease[J]. Int J Biochem Cell Biol, 2007, 39(1): 44-84.
|
| 34 |
MYSAK J, PODZIMEK S, SOMMEROVA P, et al. Porphyromonas gingivalis: major periodontopathic pathogen overview[J]. J Immunol Res, 2014, 2014: 476068.
|
| 35 |
ROCHA D M, CALDAS A P, OLIVEIRA L L, et al. Saturated fatty acids trigger TLR4-mediated inflammatory response[J]. Atherosclerosis, 2016, 244: 211-215.
|
| 36 |
SONG M, JIN J J, LIM J E, et al. TLR4 mutation reduces microglial activation, increases Aβ deposits and exacerbates cognitive deficits in a mouse model of Alzheimer’s disease[J]. J Neuroinflammation, 2011, 8: 92.
|
| 37 |
NATIVEL B, COURET D, GIRAUD P, et al. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human[J]. Sci Rep, 2017, 7(1): 15789.
|
| 38 |
WU C F, LIU C W, LUO K, et al. Changes in expression of the membrane receptors CD14, MHC-Ⅱ, SR-A, and TLR4 in tissue-specific monocytes/macrophages following Porphyromonas gingivalis-LPS stimulation[J]. Inflammation, 2018, 41(2): 418-431.
|
 )
)