吉林大学学报(工学版) ›› 2024, Vol. 54 ›› Issue (11): 3114-3124.doi: 10.13229/j.cnki.jdxbgxb.20230049
• 车辆工程·机械工程 • 上一篇
MMH凝胶液滴蒸发与燃烧过程的数值仿真
- 天津大学 内燃机燃烧学国家重点实验室,天津 300072
Numerical simulation of evaporation and combustion of MMH gel droplets
Fan ZHANG( ),Ning HAN,Qing DU,Jing-qi BU,Zhi-jun PENG(
),Ning HAN,Qing DU,Jing-qi BU,Zhi-jun PENG( )
)
- State Key Laboratory of Engines,Tianjin University,Tianjin 300072,China
摘要:
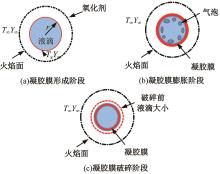
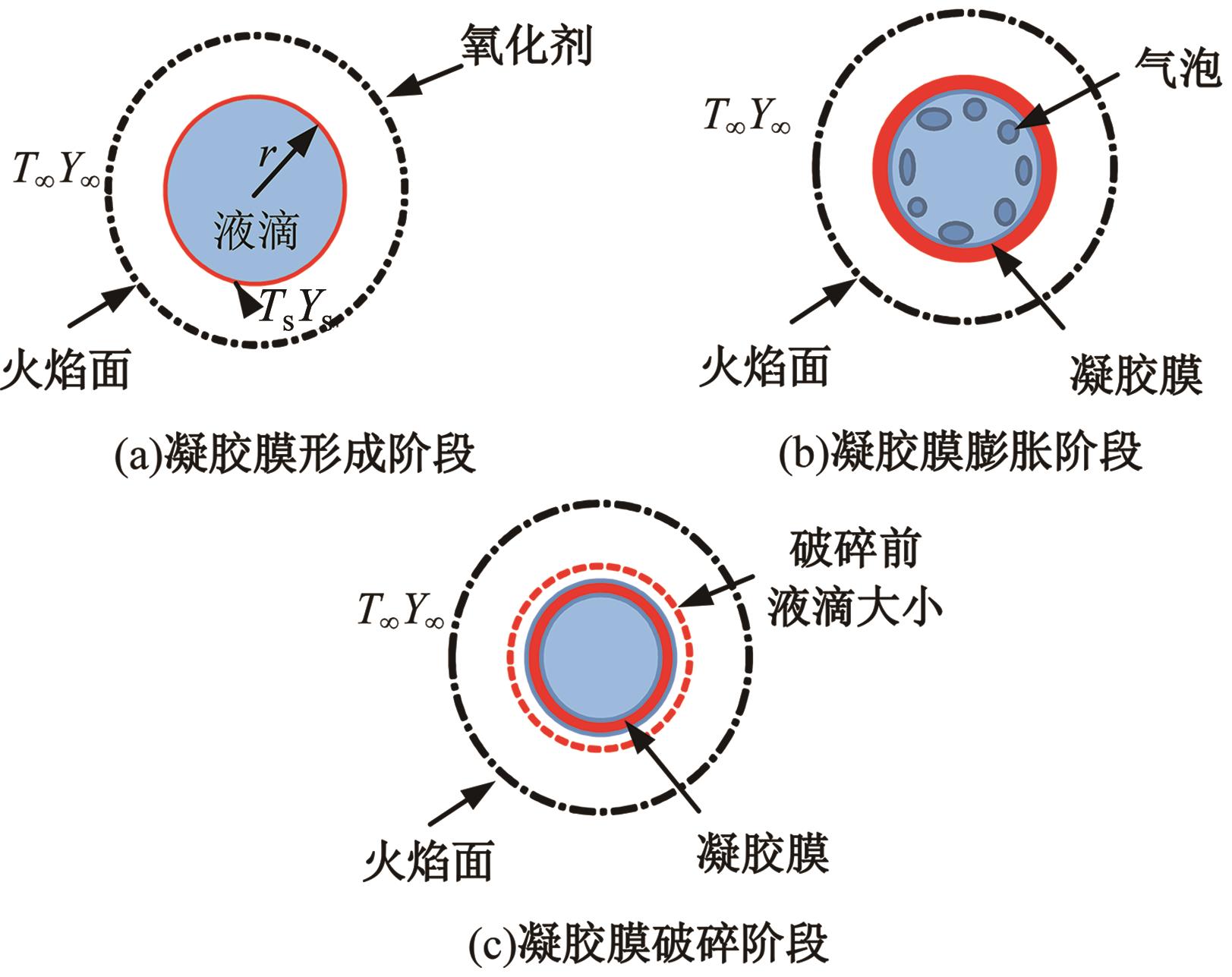
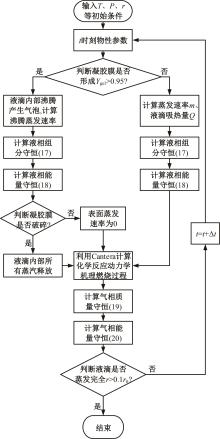
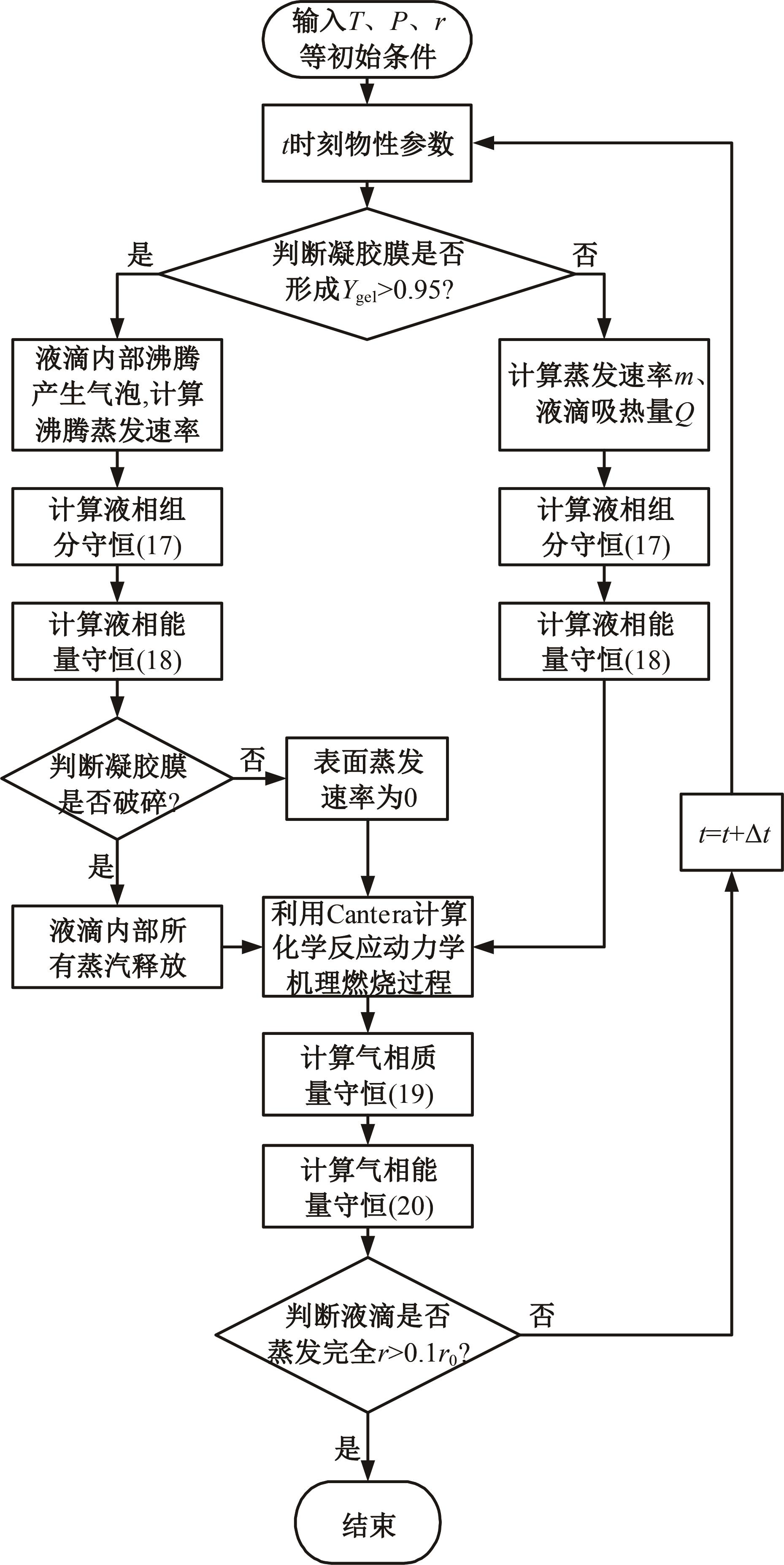
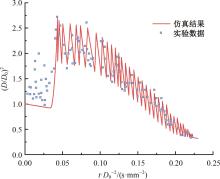
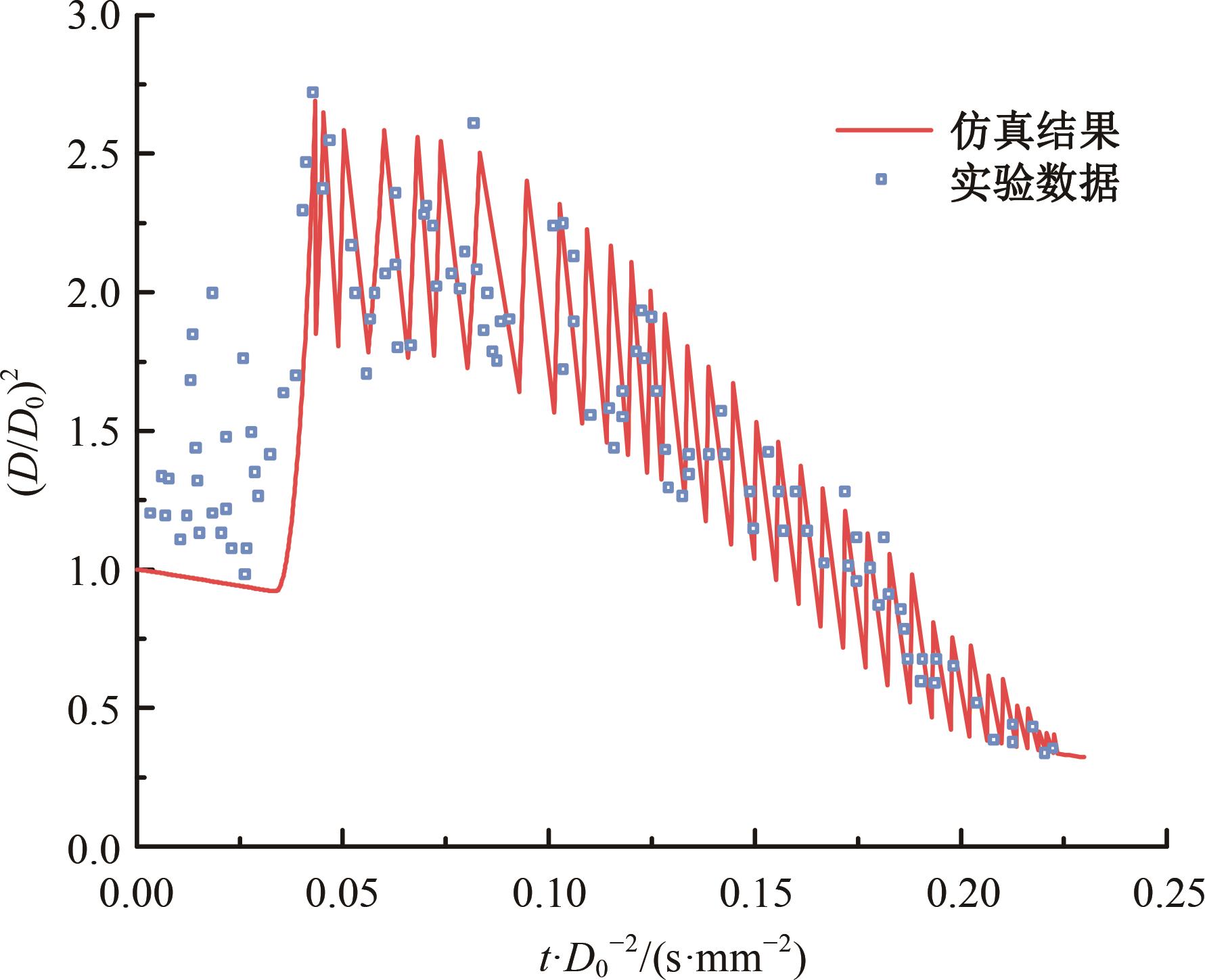
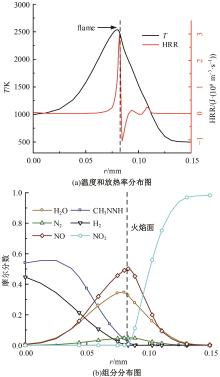
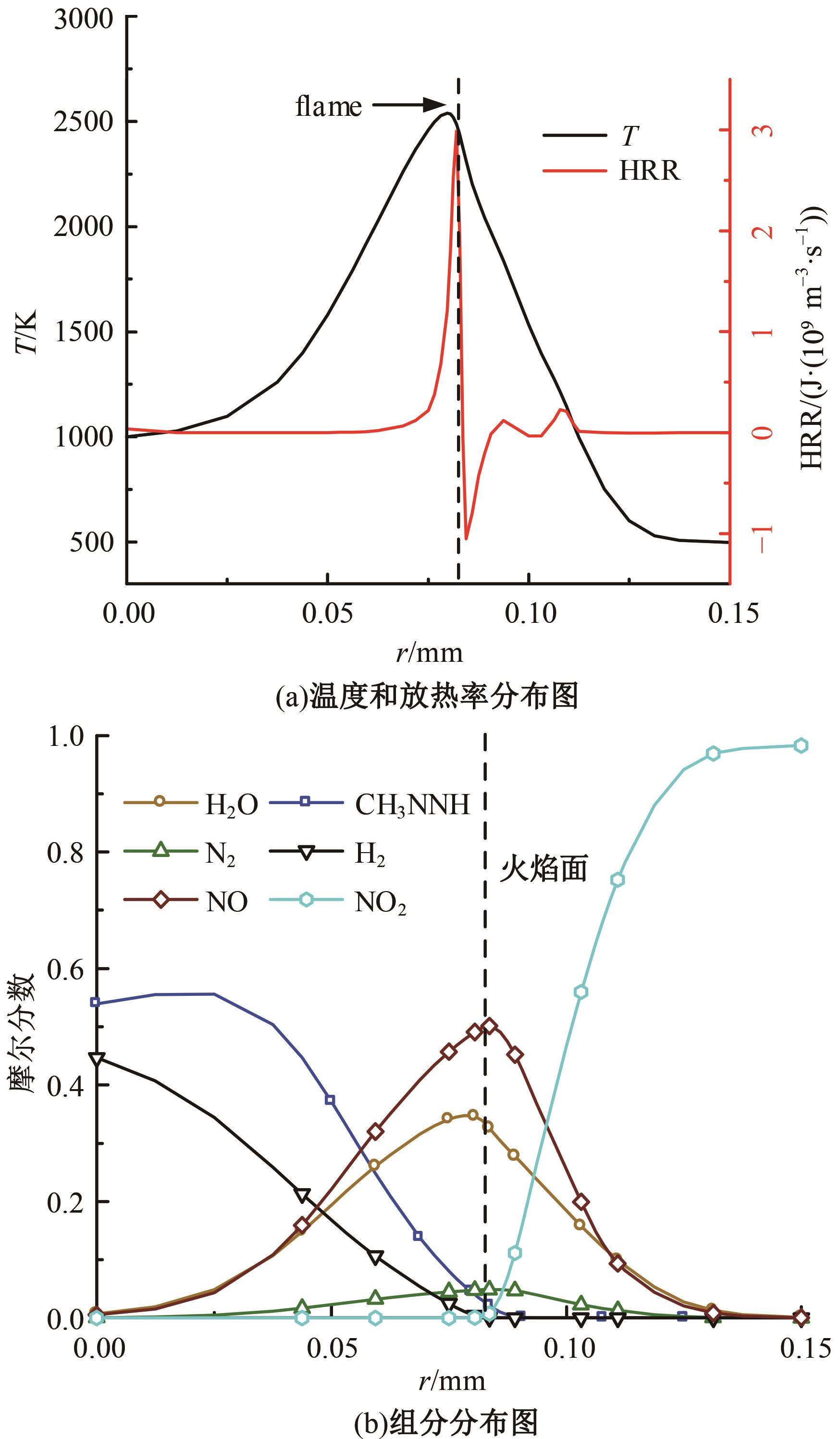
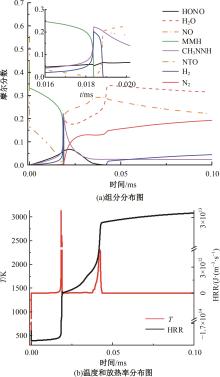
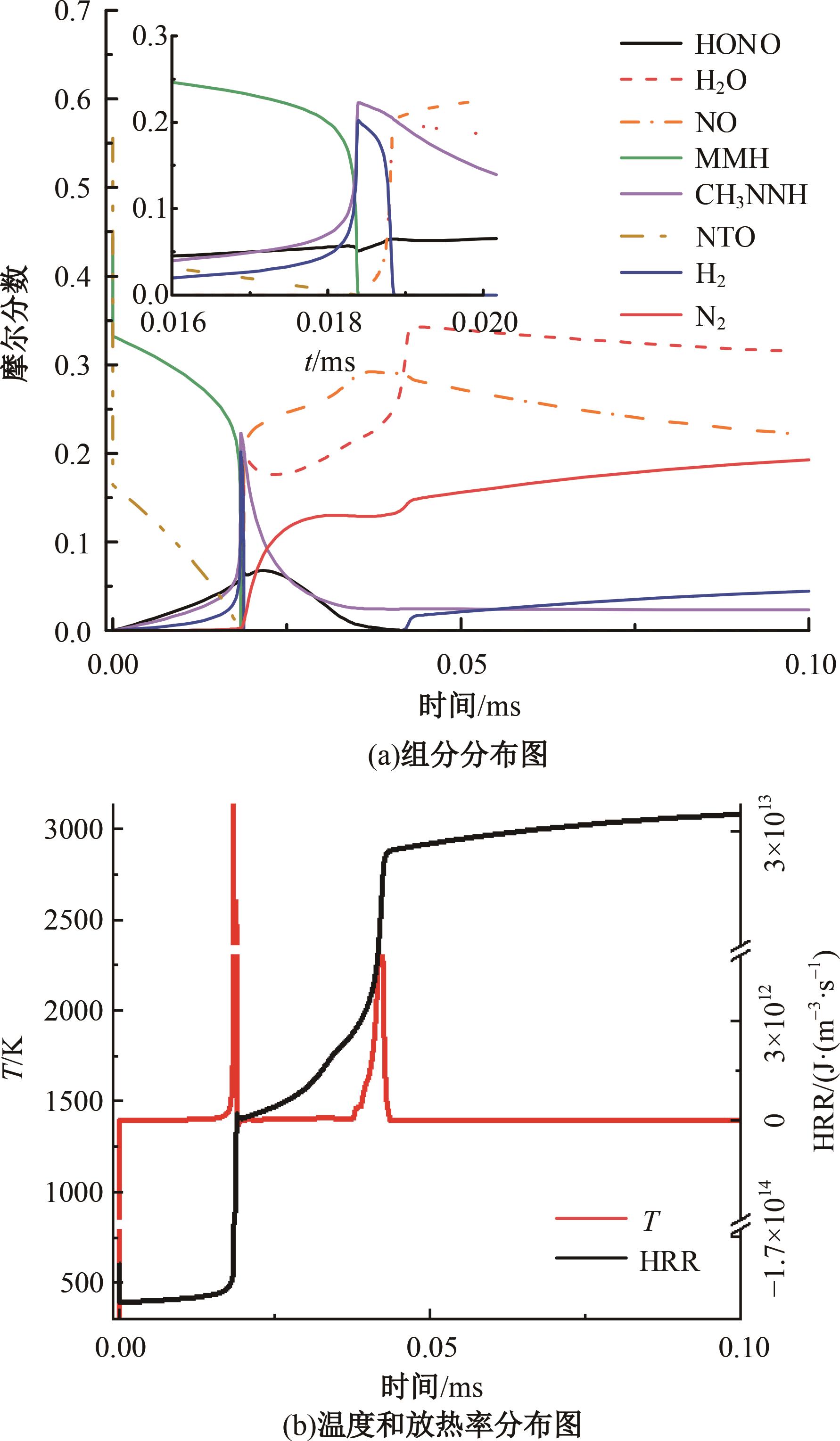
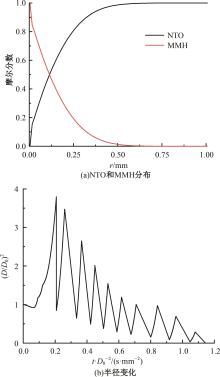
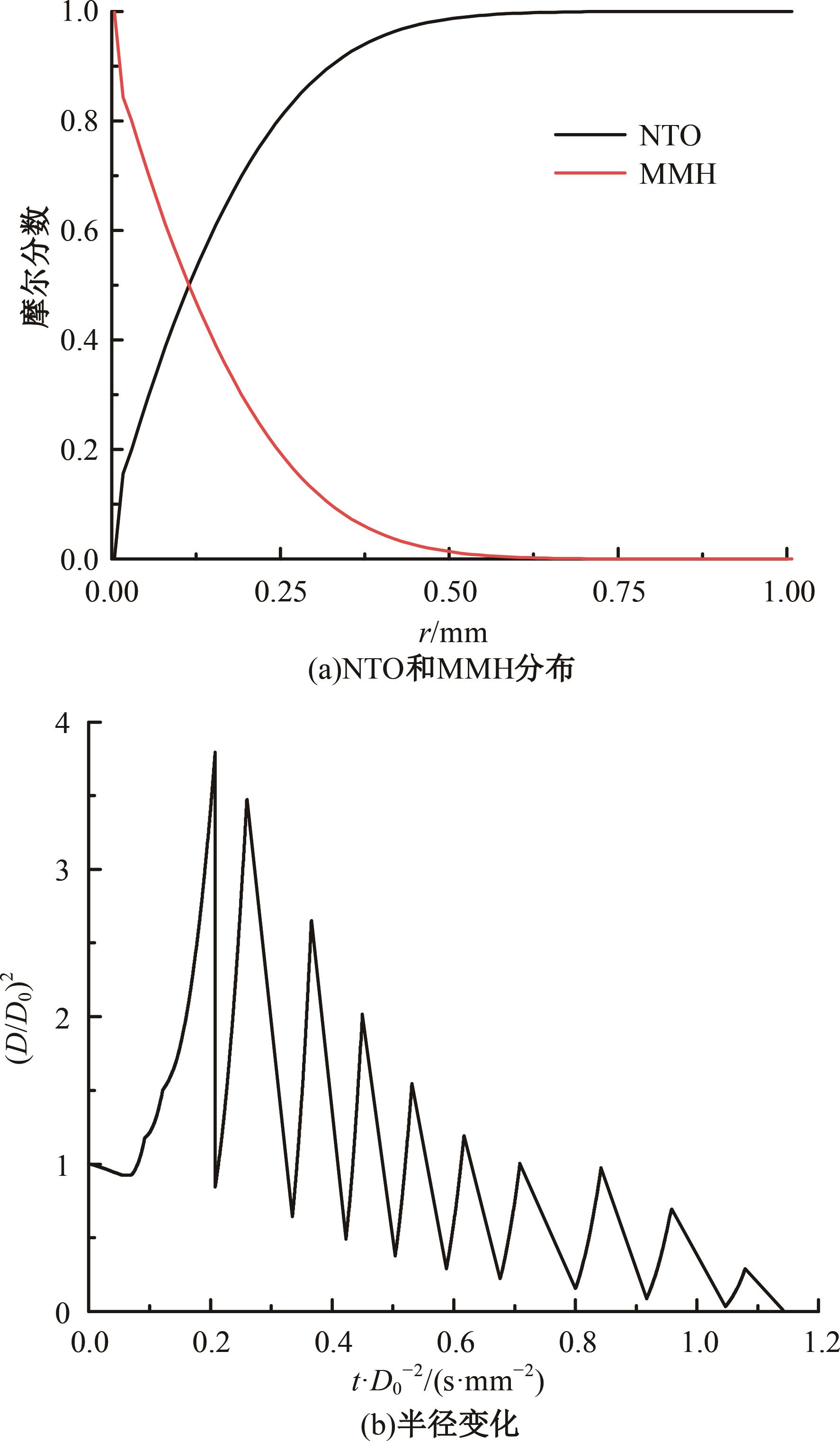
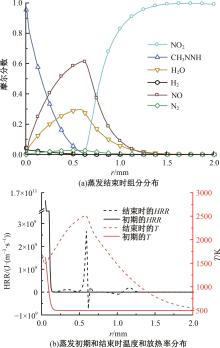
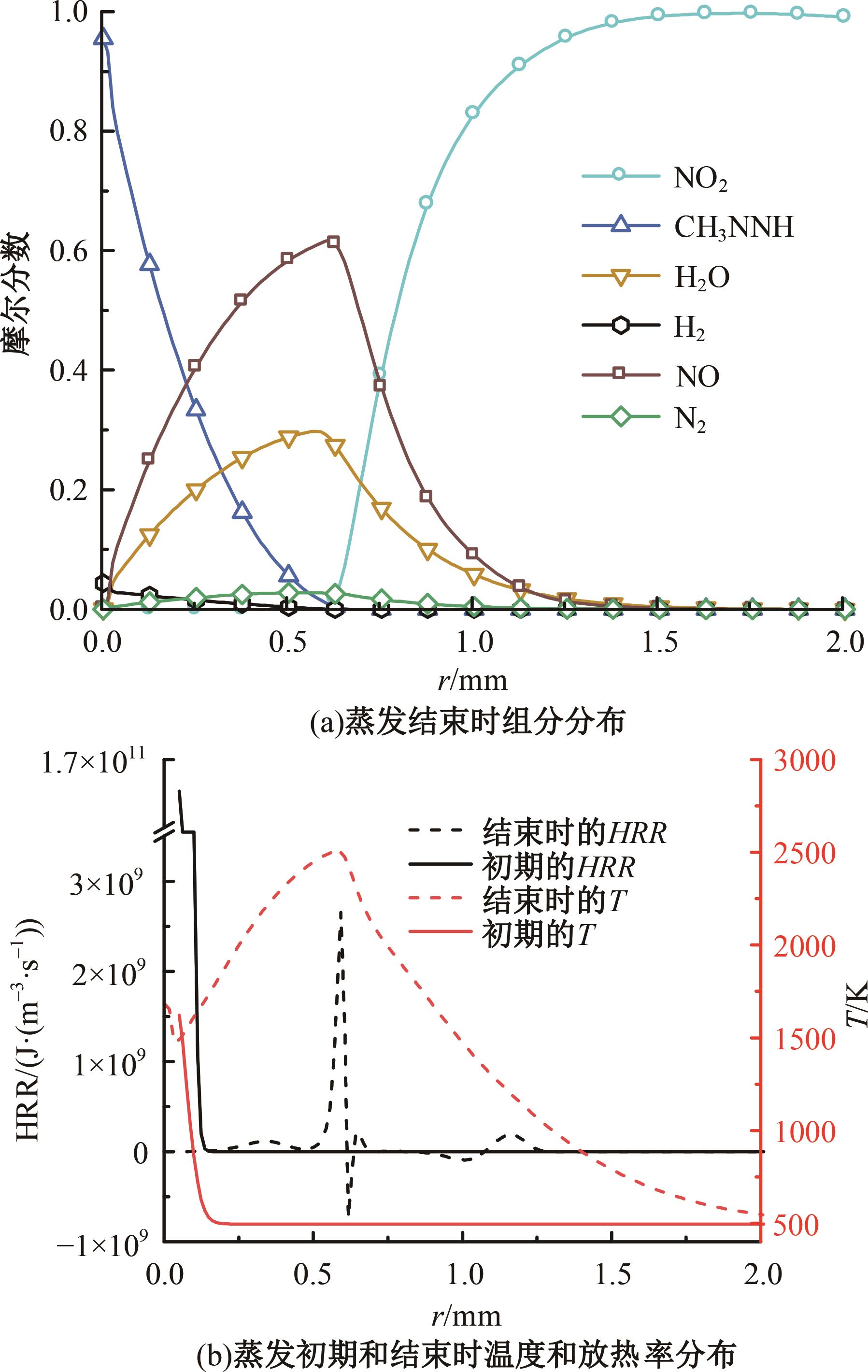
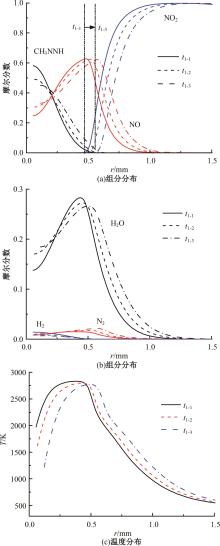
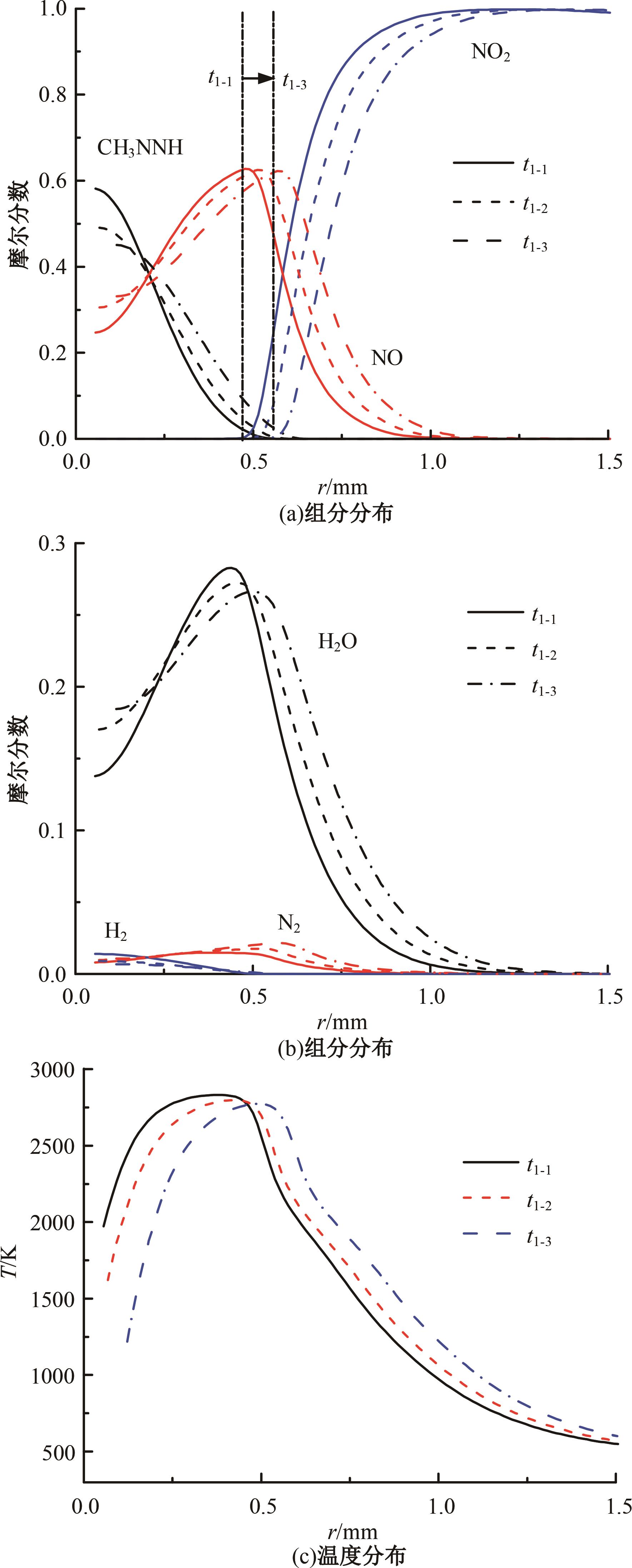
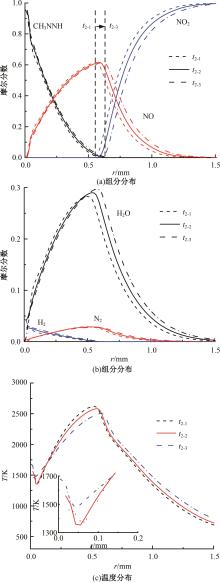
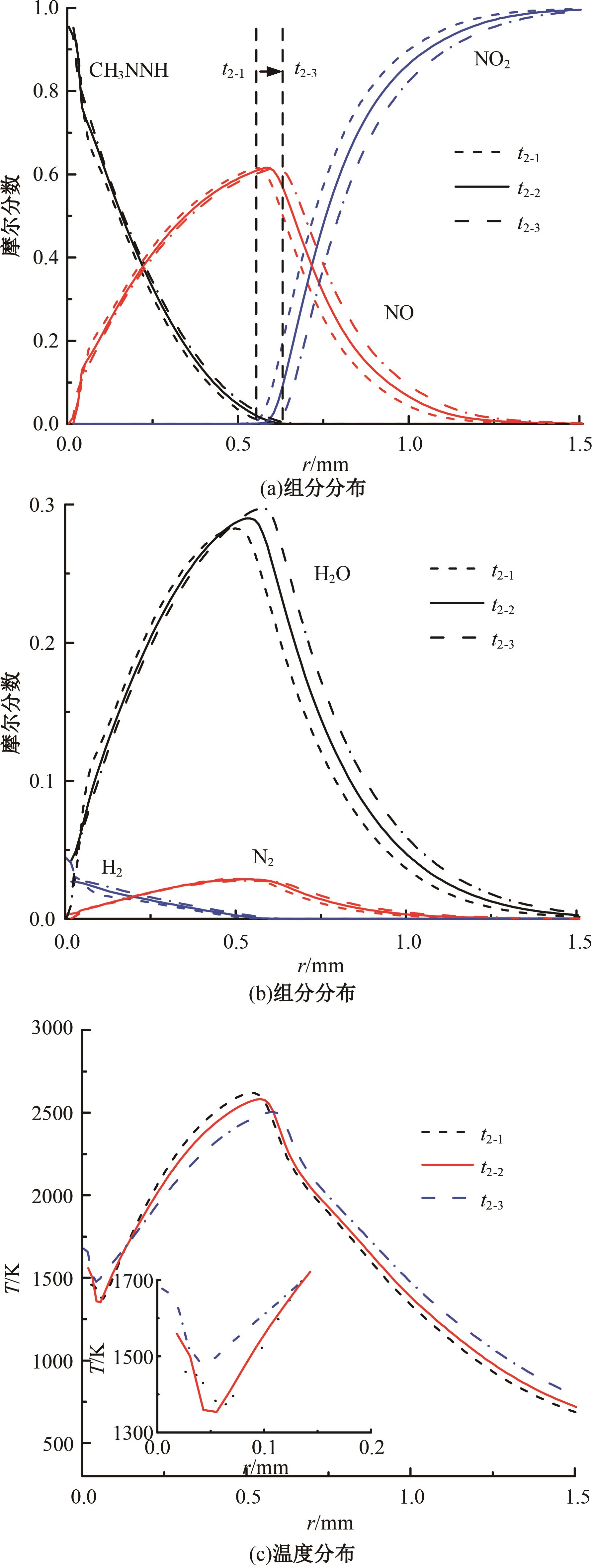
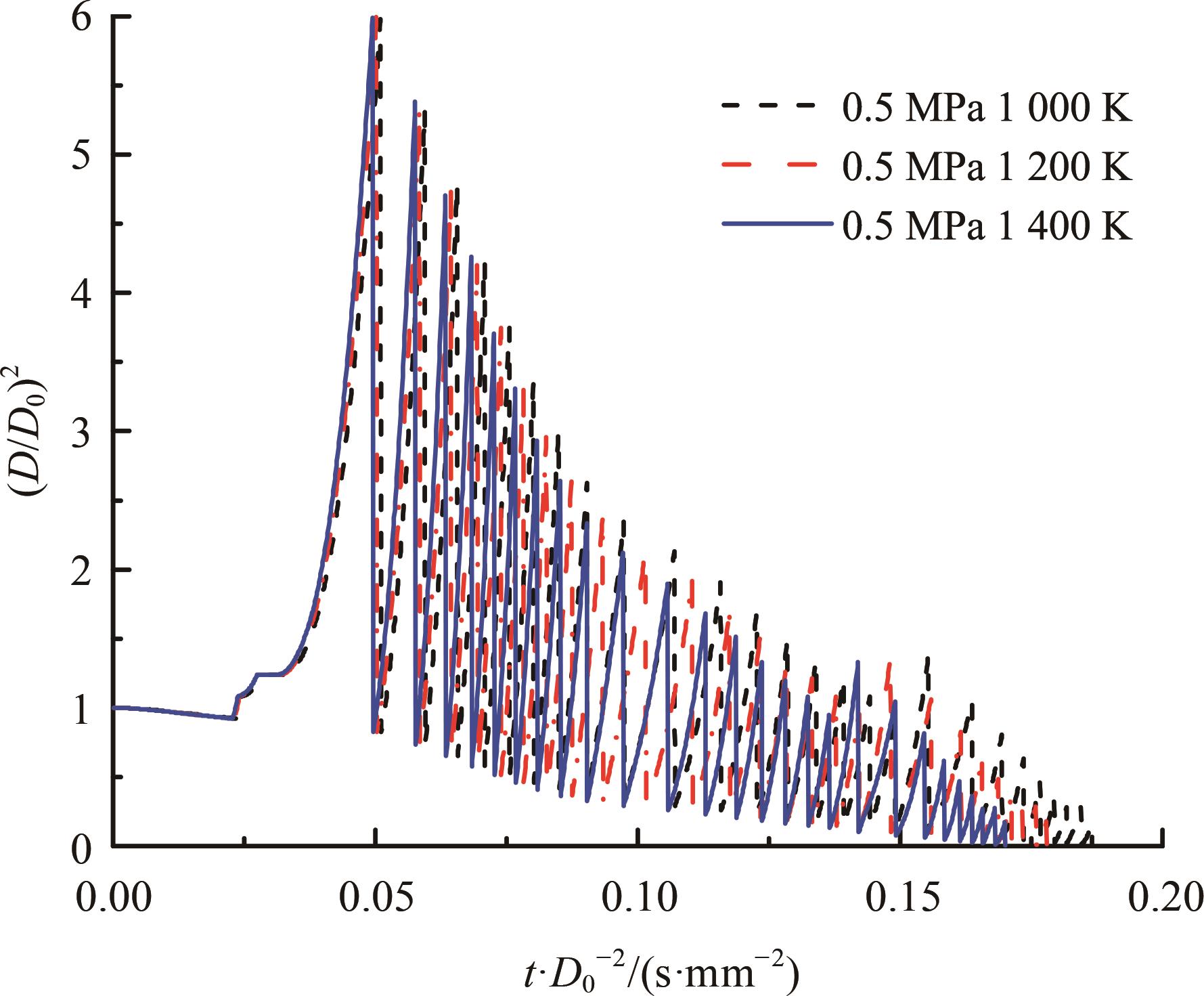
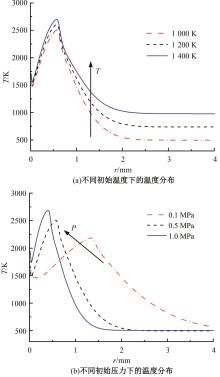
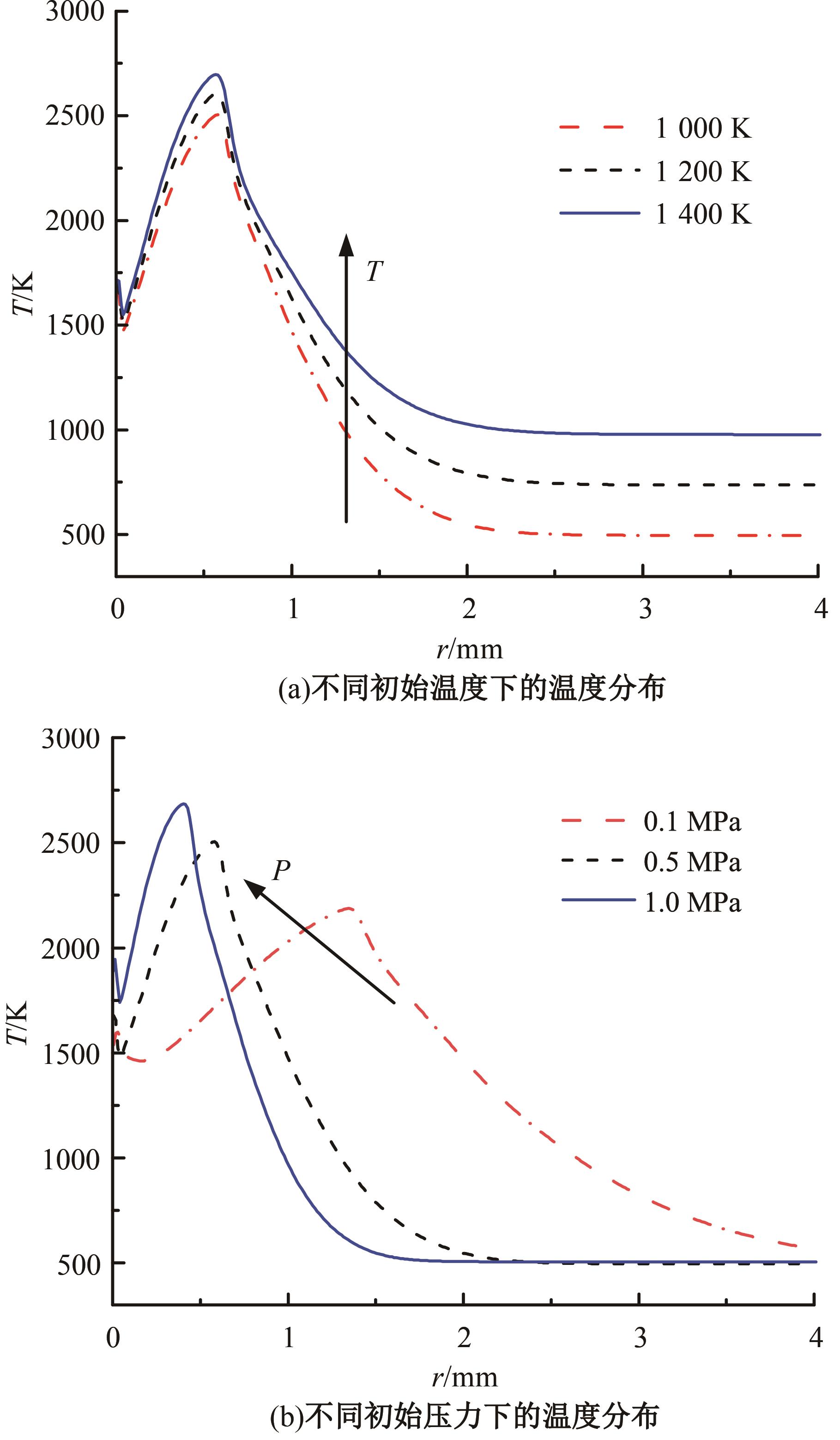
本文对耦合甲基肼(MMH)凝胶液滴蒸发模型和MMH/NTO的燃烧动力学机理进行了MMH凝胶液滴蒸发与燃烧过程的数值模拟研究。首先,研究了MMH/NTO的一维对冲火焰和低温零维化学反应动力学过程,发现MMH受热会立刻分解为CH3NNH和H2,MMH/NTO混合物存在两阶段点火现象。进一步在压力为0.5 MPa、温度为1 000 K条件下对MMH/NTO凝胶单液滴的蒸发和燃烧过程进行了一系列仿真模拟,发现了凝胶膜形成、膨胀、破碎过程,以及破碎之后释放的MMH蒸汽与环境中的NTO相互扩散形成非预混的火焰面;同时,液滴半径的变化呈现振荡现象。在凝胶液滴两次破碎时刻之间,由于MMH蒸汽的不断消耗,火焰面处温度也会随之逐渐降低;随着时间的推移,凝胶液滴膨胀-破碎的频率越快,MMH分解放热越频繁,气液交界处温度会随之升高。在一维仿真中,同样发现MMH的两阶段放热过程,NTO的分解吸热导致周围温度略有降低。最后,比较了初始温度和初始压力对燃烧过程的影响,发现环境温度越高,液滴膨胀-破碎频率越快,凝胶液滴的寿命越短;压力增大,火焰面更靠近液滴,与常规液滴蒸发燃烧过程类似。
中图分类号:
- V231
| 1 | Rapp D, Zurawski R. Characterization of aluminum/RP-1 gel propellant properties[C]∥ 24th Joint Propulsion Conference, Boston, USA, 1988: 2821. |
| 2 | Haddad A, Natan B, Arieli R. The performance of a boron-loaded gel-fuel ramjet[J]. Progress in Propulsion Physics, 2011, 2: 499-518. |
| 3 | Natan B, Rahimi S. The status of gel propellants in year 2000[J]. International Journal of Energetic Materials and Chemical Propulsion, 2002, 5: 1-6. |
| 4 | 杨大力. 凝胶单液滴蒸发燃烧特性试验研究[D]. 长沙: 国防科学技术大学空天科学学院, 2015. |
| Yang Da-li. Experimental study on evaporative combustion characteristics of gel single droplet[D]. Changsha: School of Aerospace Science, National University of Defense Technology, 2015. | |
| 5 | Solomon Y, Natan B, Cohen Y. Combustion of gel fuels based on organic gellants[J]. Combustion and Flame, 2009, 156(1): 261-268. |
| 6 | Zhou Z F, Yin J, Chen B, et al. Liquid phase model and its coupling interaction with the ambient gas for the droplet heating and evaporation of highly volatile R134a[J]. International Journal of Heat and Mass Transfer, 2021, 166: No.120740. |
| 7 | Shen S, Che Z, Wang T, et al. A model for droplet heating and evaporation of water-in-oil emulsified fuel[J]. Fuel, 2020, 266: No.116710. |
| 8 | Abramzon B, Sirignano W A. Droplet vaporization model for spray combustion calculations[J]. International Journal of Heat and Mass Transfer, 1989, 32(9): 1605-1618. |
| 9 | 何博, 何浩波, 丰松江, 等. 液体火箭有机凝胶喷雾液滴蒸发模型及仿真研究[J]. 物理学报, 2012, 61(14): 440-450. |
| He Bo, He Hao-bo, Feng Song-jiang, et al. Droplet evaporation model and simulation of liquid rocket organogels spray[J]. Acta Physica Sinica, 2012, 61(14): 440-450. | |
| 10 | 张龙. 凝胶单液滴蒸发模型的数值研究[D]. 天津: 天津大学机械工程学院, 2014. |
| Zhang Long. Numerical study of gel single drop evaporation model[D]. Tianjin: School of Mechanical Engineering, Tianjin University, 2014. | |
| 11 | 强洪夫, 张林涛, 陈福振, 等. 基于 SPH 方法的凝胶燃料单滴微爆过程模拟[J]. 含能材料, 2017, 25(5): 372-378. |
| Qiang Hong-fu, Zhang Lin-tao, Chen Fu-zhen, et al. Simulation of single drop microdetonation process of gel fuel based on SPH method[J]. Journal of Energetic Materials, 2017, 25(5): 372-378. | |
| 12 | 何博, 聂万胜, 庄逢辰. 偏二甲肼有机凝胶液滴蒸发燃烧模型展望[J]. 化学学报, 2013, 71: 302-307. |
| He Bo, Nie Wan-sheng, Zhuang Feng-chen. Prospect of evaporative combustion model of undimethylhydrazine organic gel droplet[J]. Acta Chimica Sinica, 2013, 71: 302-307. | |
| 13 | Catoire L, Swihart M T. Thermochemistry of species produced from monomethylhydrazine in propulsion and space-related applications[J]. Journal of Propulsion and Power, 2002, 18(6): 1242-1253. |
| 14 | Catoire L, Chaumeix N, Paillard C. Chemical kinetic model for monomethylhydrazine/nitrogen tetroxide gas phase combustion and hypergolic ignition[J]. Journal of Propulsion and Power, 2004, 20(1): 87-92. |
| 15 | 巴延涛, 侯凌云, 毛晓芳, 等. 甲基肼/四氧化二氮反应化学动力学模型构建及分析[J]. 物理化学学报, 2014, 30(6): 1042-1048. |
| Ba Yan-tao, Hou Ling-yun, Mao Xiao-fang, et al. Construction and analysis of chemical kinetics model of methylhydrazine/nitrous oxide reaction[J]. Acta Physico-Chimica Sinica, 2014, 30(6): 1042-1048. | |
| 16 | Hou L, Fu P, Ba Y. Chemical mechanism of MMH/NTO and simulation in a small liquid rocket engine[J]. Combustion Science and Technology, 2019, 191(12): 2208-2225. |
| 17 | Rao P M, Raghavan V, Velusamy K, et al. Modeling of quasi-steady sodium droplet combustion in convective environment[J]. International Journal of Heat and Mass Transfer, 2012, 55(4): 734-743. |
| 18 | Chen W, Gao R, Sun J, et al. Modeling of an isolated liquid hydrogen droplet evaporation and combustion[J]. Cryogenics, 2018, 96: 151-158. |
| 19 | Higham D J, Higham N J. MATLAB Guide[M]. Philadelphia: Society for Industrial and Applied Mathematics, 2016. |
| 20 | Goodwin D G, Moffat H K, Speth R L. Cantera: an object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes[J]. Cantera, 2023, 3: No.8137090. |
| 21 | Stephen R T. 燃烧学导论:概念与应用[M]. 姚强,李水清,王宇,译.3版.北京: 清华大学出版社, 2015. |
| 22 | 聂万胜, 何博, 苏凌宇, 等. 有机凝胶偏二甲肼液滴着火燃烧特性及影响因素实验研究[J]. 实验流体力学, 2013, 27(4): 23-31. |
| Nie Wan-sheng, He Bo, Su Ling-yu, et al. Experimental study on combustion characteristics and influencing factors of droplet of undimethylhydrazine organic gel[J]. Experimental Fluid Mechanics, 2013, 27(4): 23-31. | |
| 23 | Kee R J, Miller J A, Jefferson T H. Chemkin: a general-purpose, problem-independent, transportable, fortran chemical kinetics code package[R]. Albuquerque: Sandia National Laboratories, 1980. |
| [1] | 商蕾,杨萍,杨祥国,潘建欣,杨军,张梦如. 基于APSO-BP-PID控制的质子交换膜燃料电池热管理系统温度控制[J]. 吉林大学学报(工学版), 2024, 54(9): 2401-2413. |
| [2] | 陈贵升,罗国焱,李靓雪,黄震,李一. 柴油机颗粒捕集器孔道流场及其高原环境下噪声特性分析[J]. 吉林大学学报(工学版), 2023, 53(7): 1892-1901. |
| [3] | 王建,于威,王斌. 高原状态下甲醇替代率对柴油机燃烧与排放的影响[J]. 吉林大学学报(工学版), 2023, 53(4): 954-963. |
| [4] | 金兆辉,谷乐祺,洪伟,解方喜,尤田. 液压可变气门系统压力波动的影响分析[J]. 吉林大学学报(工学版), 2022, 52(4): 773-780. |
| [5] | 张岩,刘玮,张树勇,裴毅强,董蒙蒙,秦静. 二/四冲程可变柴油机燃烧室热负荷的改善[J]. 吉林大学学报(工学版), 2022, 52(3): 504-514. |
| [6] | 赵文伯,李玉洁,邓俊,李理光,吴志军. 针阀运动规律及其对喷嘴内流和喷雾特性影响[J]. 吉林大学学报(工学版), 2022, 52(10): 2234-2243. |
| [7] | 赵庆武,程勇,杨雪,王宁. 高重频纳秒脉冲放电点火系统设计[J]. 吉林大学学报(工学版), 2021, 51(2): 414-421. |
| [8] | 李志军,刘浩,张立鹏,李振国,邵元凯,李智洋. 过滤壁结构对颗粒捕集器深床过滤影响的模拟[J]. 吉林大学学报(工学版), 2021, 51(2): 422-434. |
| [9] | 王忠,李游,张美娟,刘帅,李瑞娜,赵怀北. 柴油机排气阶段颗粒碰撞过程动力学特征分析[J]. 吉林大学学报(工学版), 2021, 51(1): 39-48. |
| [10] | 胡云峰,丁一桐,赵志欣,蒋冰晶,高金武. 柴油发动机燃烧过程数据驱动建模与滚动优化控制[J]. 吉林大学学报(工学版), 2021, 51(1): 49-62. |
| [11] | 王建,许鑫,顾晗,张多军,刘胜吉. 基于排气热管理的柴油机氧化催化器升温特性[J]. 吉林大学学报(工学版), 2020, 50(2): 408-416. |
| [12] | 宋昌庆,陈文淼,李君,曲大为,崔昊. 不同当量比下单双点火对天然气燃烧特性的影响[J]. 吉林大学学报(工学版), 2019, 49(6): 1929-1935. |
| [13] | 朱一骁,何小民,金义. 联焰板宽度对单凹腔驻涡燃烧室流线形态的影响[J]. 吉林大学学报(工学版), 2019, 49(6): 1936-1944. |
| [14] | 刘长铖,刘忠长,田径,许允,杨泽宇. 重型增压柴油机燃烧过程中的缸内㶲损失[J]. 吉林大学学报(工学版), 2019, 49(6): 1911-1919. |
| [15] | 胡潇宇,李国祥,白书战,孙柯,李思远. 考虑加热面粗糙度和材料的沸腾换热修正模型[J]. 吉林大学学报(工学版), 2019, 49(6): 1945-1950. |
|
||